Hysterothylacium haze ( Machida, Takahashi et Masuuchi, 1978 ) Deardorff et Overstreet, 1981
|
publication ID |
https://doi.org/ 10.14411/fp.2018.016 |
|
publication LSID |
lsid:zoobank.org:pub:BF143B96-4194-4DF7-838C-99EB047203E6 |
|
DOI |
https://doi.org/10.5281/zenodo.8178661 |
|
persistent identifier |
https://treatment.plazi.org/id/0382671D-FFA9-D617-FF08-F8968BC8E1D0 |
|
treatment provided by |
Felipe |
|
scientific name |
Hysterothylacium haze ( Machida, Takahashi et Masuuchi, 1978 ) Deardorff et Overstreet, 1981 |
| status |
|
Hysterothylacium haze ( Machida, Takahashi et Masuuchi, 1978) Deardorff et Overstreet, 1981 View in CoL
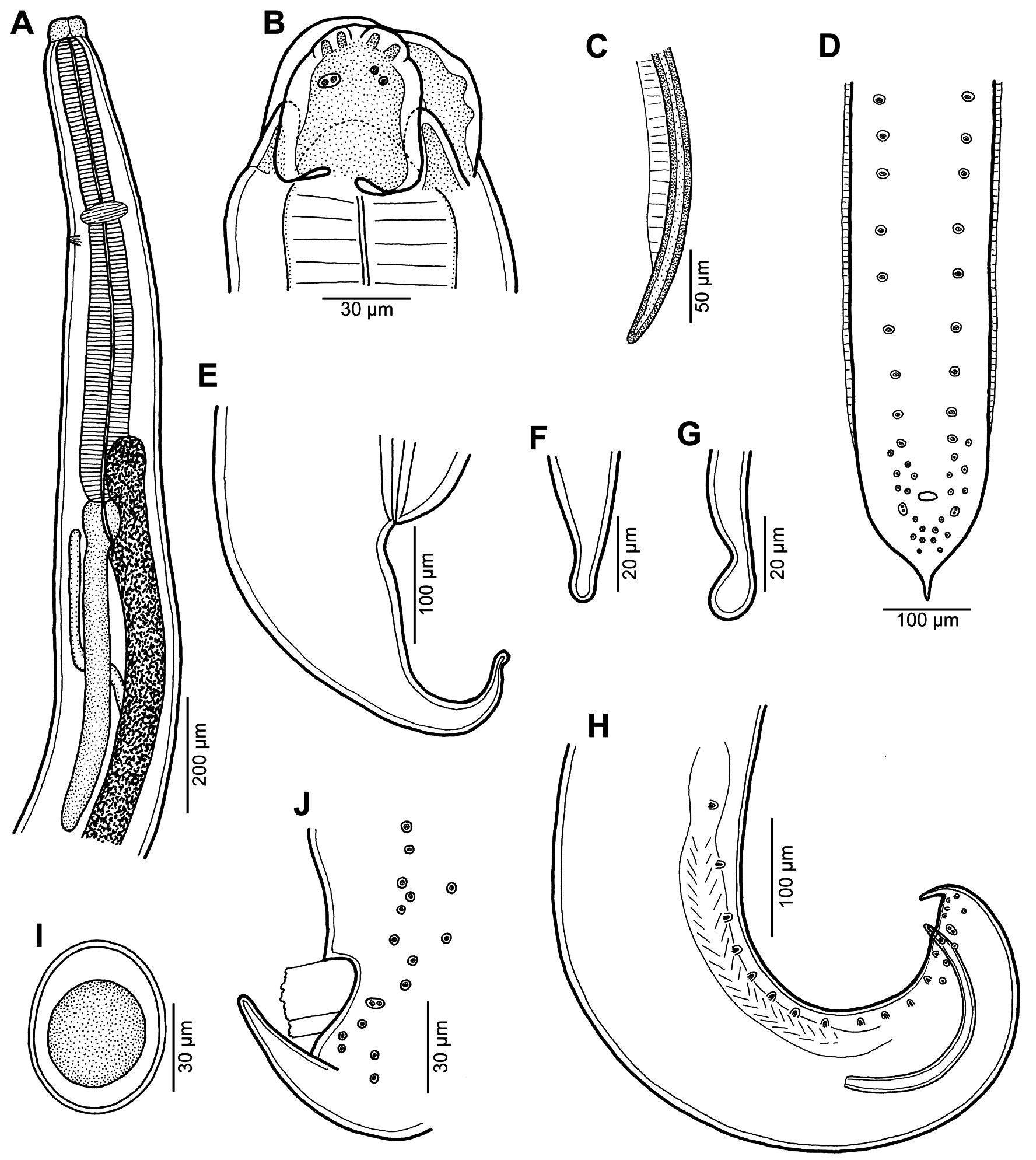
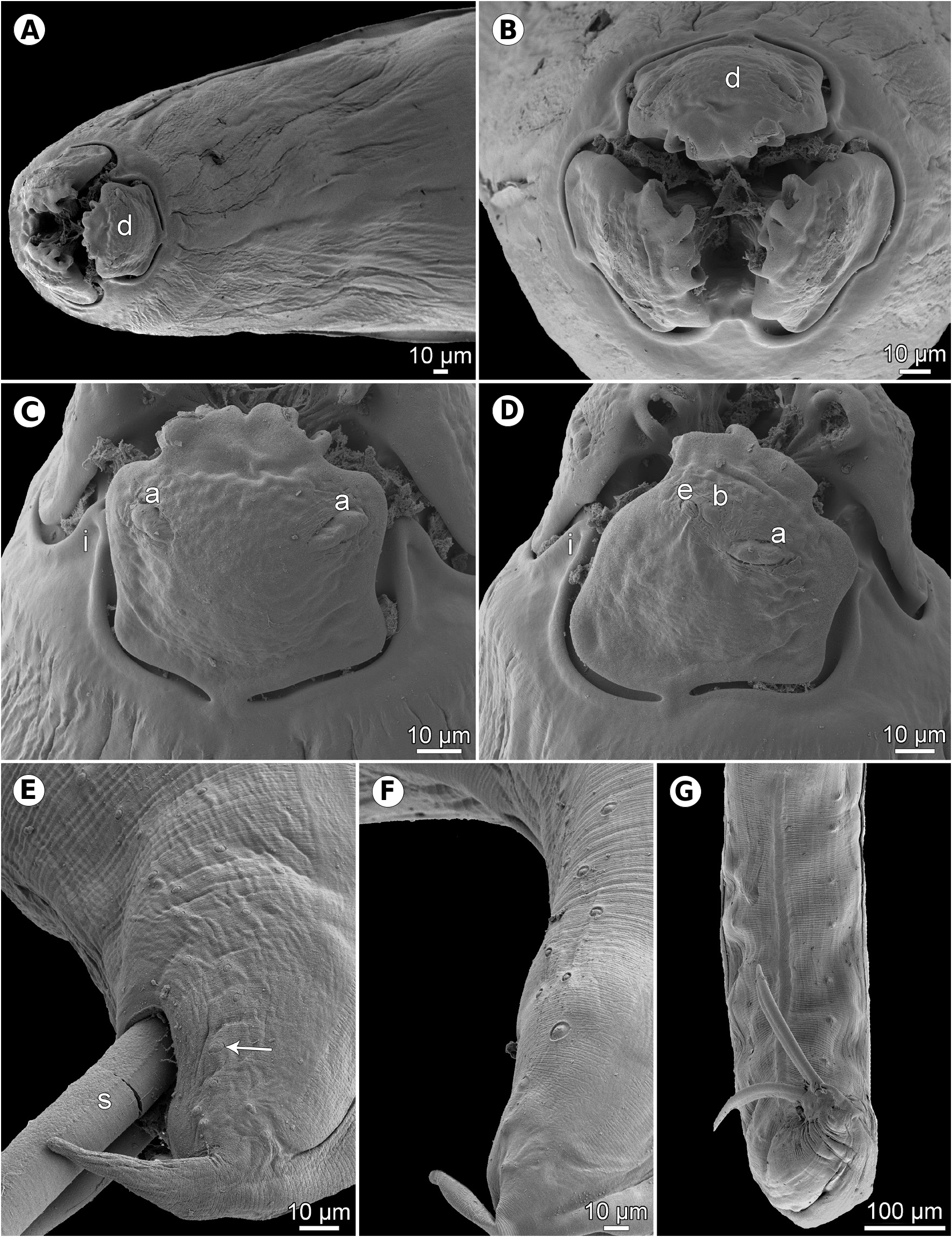
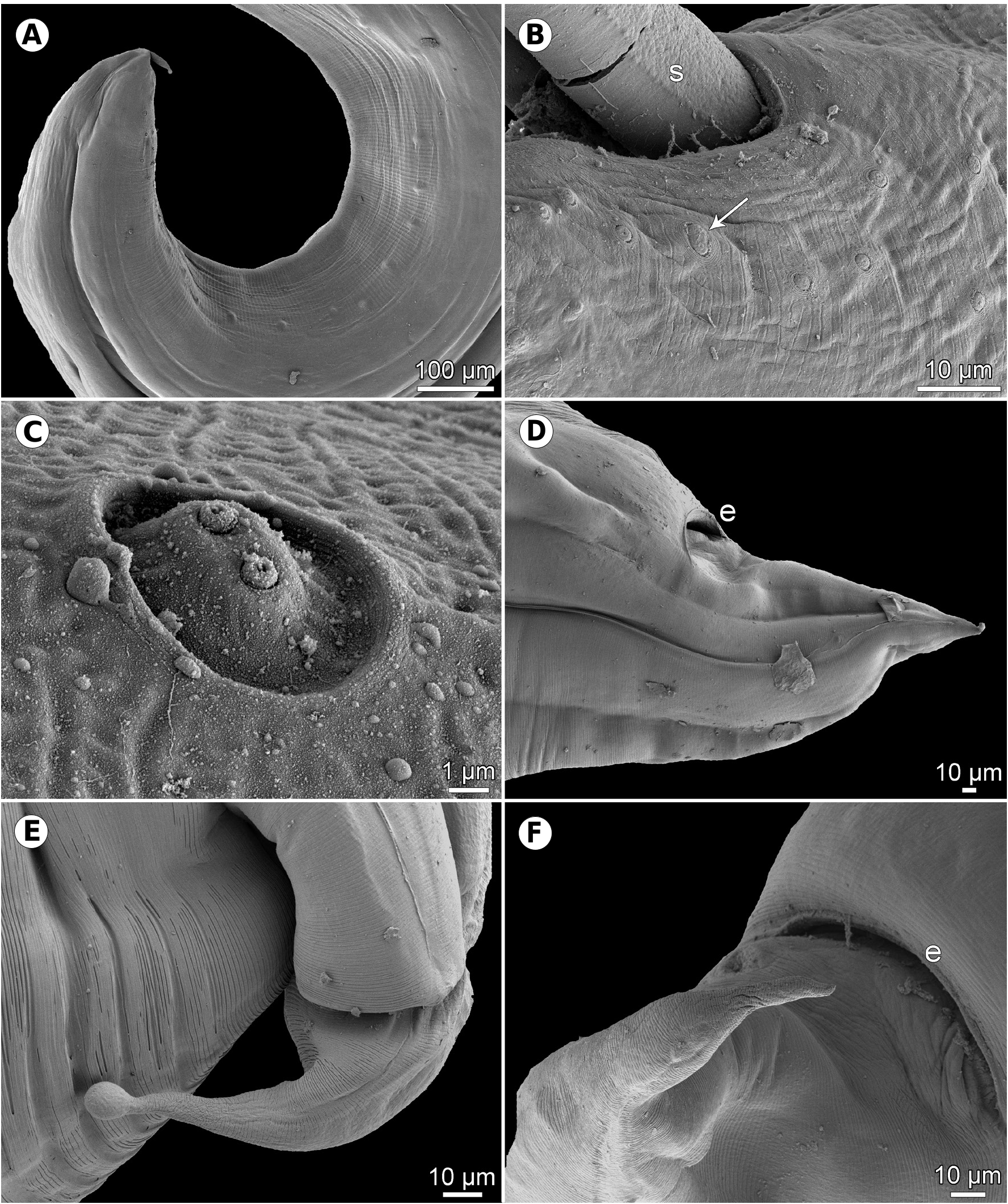
Figs. 1–3 View Fig View Fig View Fig Description. Medium-sized, whitish nematodes with slightly transversely striated cuticle ( Figs. 2E,F View Fig , 3B,D, E View Fig ). Maximum width in posterior part of body. Lips almost equal in size, slightly longer than wide, with wide lateral flanges and narrow bases; pulp with 2 anteriorly protruding projections, each divided into 2 lobes. Dorsal lip with 2 subdorsal double papillae; each subventral lip with 1 double subventral papilla, one small single papilla and amphid situated laterally ( Figs. 1B View Fig , 2A–D View Fig ). Interlabia well developed, about 1/2 length of lips ( Figs. 1B View Fig , 2A–D View Fig ). Lateral alae very narrow, starting slightly posterior to level of lip bases, extending posteriorly in both sexes as cordons almost to end of tail ( Figs. 2A,E–G View Fig , 3A,D,E View Fig ). Oesophagus long, slightly broader posteriorly. Nerve ring encircles oesophagus at its anterior part. Ventriculus small, almost spherical; ventricular appendix long, narrow. Intestinal caecum short, wide ( Fig. 1A View Fig ). Excretory pore just posterior to nerve ring. Tail of both sexes conical.
Male (7 specimens). Length of body 7.5–19.2 mm, maximum width 204–544; width just posterior to base of lips 75–150. Lips 57–87 long; length of interlabia 27–39. Length of oesophagus 789–1,700, representing 9–12% of body length; maximum width 60–150. Nerve ring and excretory pore 367–544 and 408–707, respectively, from anterior extremity. Ventriculus 66–122 × 63–150; ventricular appendix 422–1,645 long, maximum width 45–109. Intestinal caecum 159–571 long, maximum width 51–163. Caecum to ventricular appendix length ratio 1: 2–4. Posterior end of body curved ventrally. Spicules equal, alate except for pointed tip, 381–516 long, representing 2.4–5.5% of body length ( Fig. 1C,H View Fig ). Total of 21–29 pairs of small subventral papillae present, 15–20 being preanals, 2 adanals and 6 postanals; papillae of several posteriormost preanal and postanal pairs very small, arranged in 2 longitudinal rows; papillae of first postanal pair doubled ( Fig. 1D,H,J View Fig ). Tail conical, 66–129 long, markedly narrowed at its distal portion; tip rounded ( Figs. 1D,H,J View Fig , 2E–G View Fig , 3A View Fig ).
Female (5 gravid specimens). Length of body 12.6– 21.6 mm, maximum width 422–734; width just posterior to base of lips 122–177. Lips 75–111 long. Length of interlabia 39–48. Length of oesophagus 1.12–1.85 mm, representing 6–13% of body length; maximum width 90–177. Nerve ring and excretory pore 435–898 and 490–1,047, respectively, from anterior extremity. Ventriculus 57–122 × 57–150; ventricular appendix 503–1,727 long, maximum width 82–150. Intestinal caecum 204–530 long, maximum width 95–150. Caecum to ventricular appendix length ratio 1: 2–4. Vulva pre-equatorial, 4.5–6.7 mm from anterior end of body, at 25–36% of body length. Vagina directed posteriorly from vulva. Uterus contains numerous oval, thin-walled, unembryonated eggs 60–66 long and 51–60 wide ( Fig. 1I View Fig ). Tail conical, 204–258 long; tip rounded, sometimes bulbously expanded ( Figs. 1E–G View Fig , 3D–F View Fig ).
Host: Yellowfin goby, Acanthogobius flavimanus ( Gobiidae , Perciformes ).
Site of infection: Body cavity.
Locality : Lake Hamana (brackish-water), Hamamatsu, Shizuoka Prefecture, Honshu, Japan (collected 16 November 2009) .
Prevalence and intensity: 40% (4 fish infected, 15.2–21.0 cm in total length [TL]/10 fish examined, 14.5–21.0 cm TL); 1–43 (mean 12) nematode specimens (adults and larvae).
Deposition of voucher specimens: IPCAS N-1152.
Remarks. Thynnascaris (= Hysterothylacium ) haze was described by Machida et al. (1978) from A. flavimanus in Tokyo Bay (reported as the Bay of Tokyo), where it was associated with mass mortalities of this fish during summer seasons in 1973–1975 ( Takahashi et al. 1976, 1977). Based on specimens collected from A. flavimanus captured in a brackish-water region of Lake Hamana, Shizuoka Prefecture, Yoshinaga et al. (1988, 1989a,b) studied in detail the morphology of this nematode, including the morphogenesis of larvae, and carried out some experimental infections of yellowfin goby and aquatic invertebrates to elucidate its life cycle and transmission.
Results of the present study, especially the use of SEM, made it possible to study in detail some taxonomically important but previously insufficiently documented or unreported morphological features in H. haze , such as amphids, the presence of postanal double papillae in the male, the exact numbers and distribution of male caudal papillae and the variability in the shape of the female tail tip.
The life cycle pattern of H. haze markedly differs from those of other congeners in that the adult worms are present and the oviposition occurs in the body cavity, rather than in the intestine, of the definitive host ( Machida et al. 1978, Yoshinaga et al. 1988, 1989a). This phenomenon in H. haze was considered by Anderson (1988) to be an example of extreme precocity in which the fish intermediate host has also become a “final host” (correctly the definitive host in the conception of Odening 1976). According to Moravec (2013), another case of precocity in anisakid nematodes is apparently the record of adults of Hysterothylacium sp. in marine prawns Pandalus borealis Krøyer by Margolis and Butler (1954).
However, the present knowledge of the development and transmission of Hysterothylacium spp. is still fragmentary ( Anderson 2000). Therefore, it cannot be excluded that H. haze exhibits a variable development with alternative cycles, similar to that in Cucullanus truttae Fabricius, 1794 (see Moravec 2013), where the development may be either with the participation of the obligate intermediate host ( A. flavimanus ) and the definitive host (unknown fish species), or the complete development is realised in A. flavimanus. In such a case, A. flavimanus would be an isochronous polyvalent host ( Odening 1976).
According to Machida et al. (1978) and Yoshinaga et al. (1989a), the eggs of H. haze deposited in the body cavity are released into the water with the death of infected gobies where they may infect other gobies directly, or indirectly through invertebrate hosts. However, it is possible that infected gobies, harbouring H. haze larvae and adults, are eaten by some predatory fish species, which may then serve as a definitive or a postcyclic host. Taking into account this presumption, more attention should be paid to the transmission of this parasite in the localities where H. haze occurs in gobies. Some ecological aspects of H. haze were reported by Yoshinaga (1992).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.