Litoria balatus, Rowley & Mahony & Hines & Myers & Price & Shea & Donnellan, 2021
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5071.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:E695DE38-387E-41E0-8188-532A907C3BB1 |
|
DOI |
https://doi.org/10.5281/zenodo.5725374 |
|
persistent identifier |
https://treatment.plazi.org/id/3464AB2A-5717-FFF1-FF3E-FC77FCAC7E84 |
|
treatment provided by |
Plazi |
|
scientific name |
Litoria balatus |
| status |
sp. nov. |
Litoria balatus sp. nov.
Slender Bleating Tree Frog
Figs 11 View FIGURE 11 , 12 View FIGURE 12
Holotype. QM J91105 View Materials . An adult male collected from a small drainage in the northeastern corner of Anstead Bushland Reserve, near the junction of Mt Crosby and Hawkesbury Roads , Anstead , south-eastern Queensland (-27.5406, 152.8614) by Harry B. Hines on 2 February 2010. GoogleMaps
Material examined. See Supplementary Table S1 View TABLE 1 for details of all material examined.
Dimensions of holotype (mm). SVL 36.3 ; HL 10.2; HW 10.6; IND 2.4; EN 3.4; ED 3.5; IOD 6.4; TD 2.1; FLL 6.7; Fin3L 8.9; TL 14.8; Toe4L 13.7 .
Diagnosis. Litoria balatus sp. nov. is distinguished from all species in the Litoria rubella group by a combination of (1) adult body size 26–44 mm in males and 33–43 mm in females, (2) relatively slender build, (3) the presence of a single, continuous, irregularly edged, dark brown dorsal band, (4) the absence of light spots on the dorsum, (5) lack of a well-defined pale mid-dorsal stripe, (6) absence of distinctive pale markings above the groin, vent and along lower leg, (7) presence of a distinct dorsolateral line continuing to groin, and (8) adult males having a vocal sac that is black.
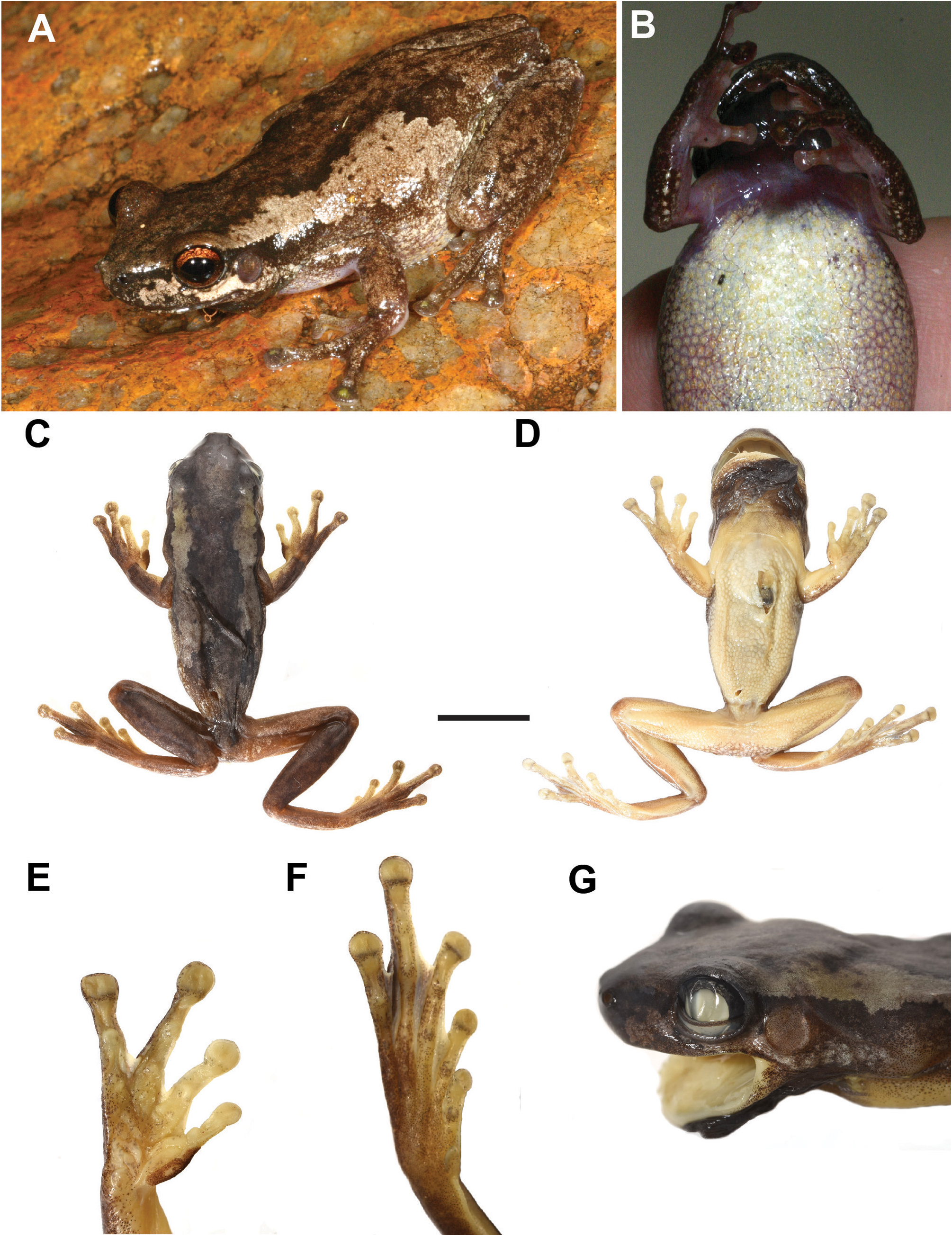
Description of holotype. Habitus slender; head widest at eyes, slightly wider than long (HW/HL 1.04); snout rounded in lateral and dorsal profiles; nostrils prominent in dorsal profile; vomerine teeth in single row running laterally anteriorly to choanae. Tympanum circular and clearly visible, about half the diameter of the eye (TD/ED 0.61).
Fingers and toes with prominent terminal discs; fingers with basal webbing, toes half webbed. Relative lengths of fingers 3>4>2>1; of toes 4>5=3>2>1. Sub-articular tubercles present under fingers and toes but not prominent; inner metatarsal tubercles present and prominent, approximately one third the length of first toe. Nuptial pad oval, restricted to dorsal surface of proximal half of first finger, comprised of small granules. Legs short (TL/SVL 0.41).
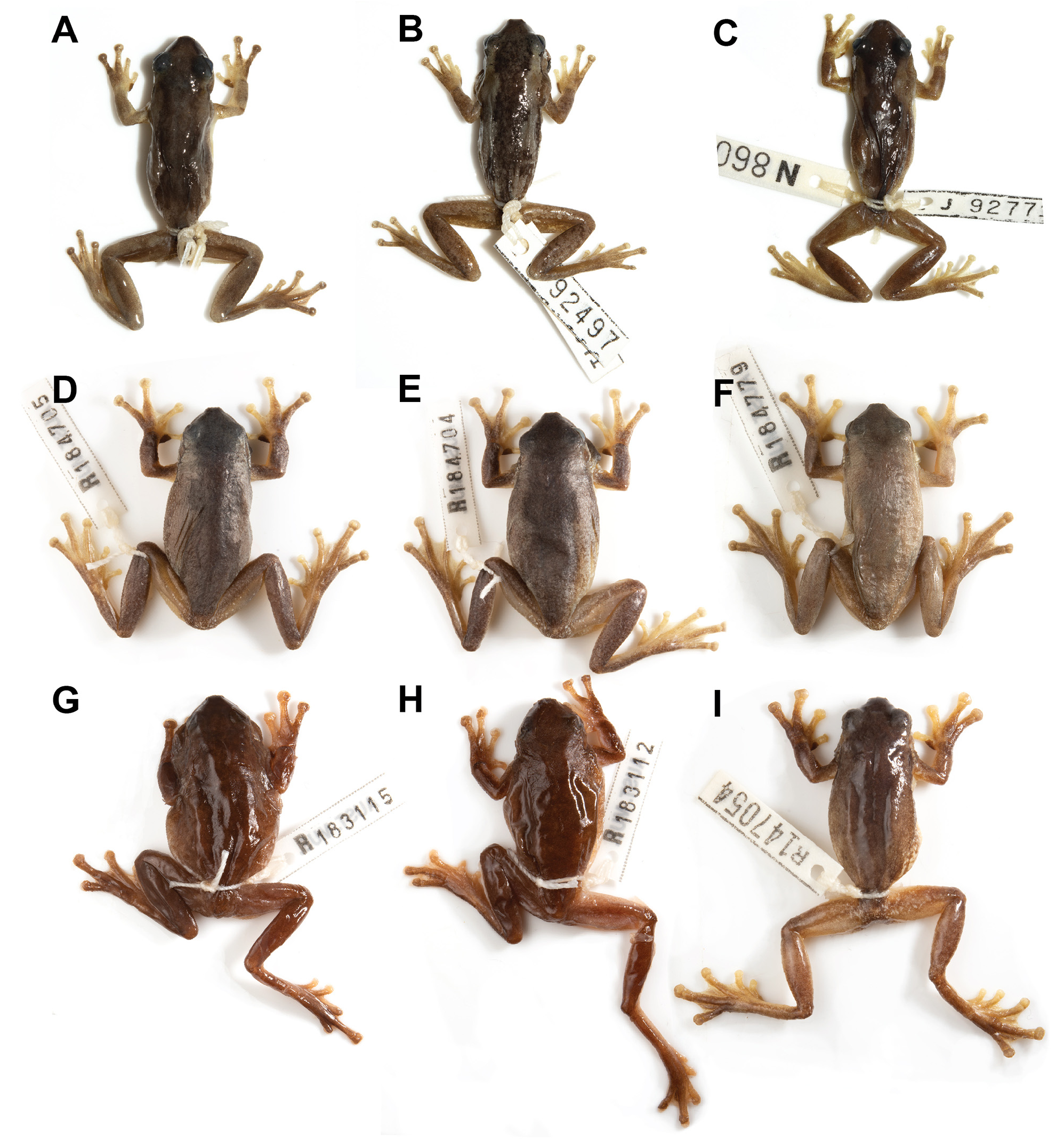
In life, all dorsal surfaces dark brown, contrasting strongly with pale ventral surfaces of body and limbs ( Fig. 11 View FIGURE 11 ). Continuous, irregularly edged, broad dark brown dorsal band from the snout to the vent, extending laterally to the dorsolateral margin. Flanks light brown. Dorsum weakly granular, becoming more granular laterally, on venter and on thighs. Upper surfaces of legs, arms and distal lower surfaces of legs and arms smooth. A dark stripe extends from snout, through eye, onto tympanum continues laterally above arm and then along lateral ventral margin of body to groin. White bar directly under eye and tympanum, immediately posterior to the tympanum. Ventral surface immaculate light cream. Single vocal sac and chin darkly pigmented, brownish-black, lower lip cream ( Fig. 11D View FIGURE 11 ).
Variation. Male SVL 26–44 mm, female SVL 33–43 mm. Summary of variation in morphometric variables for each sex is presented in Table 5 View TABLE 5 .
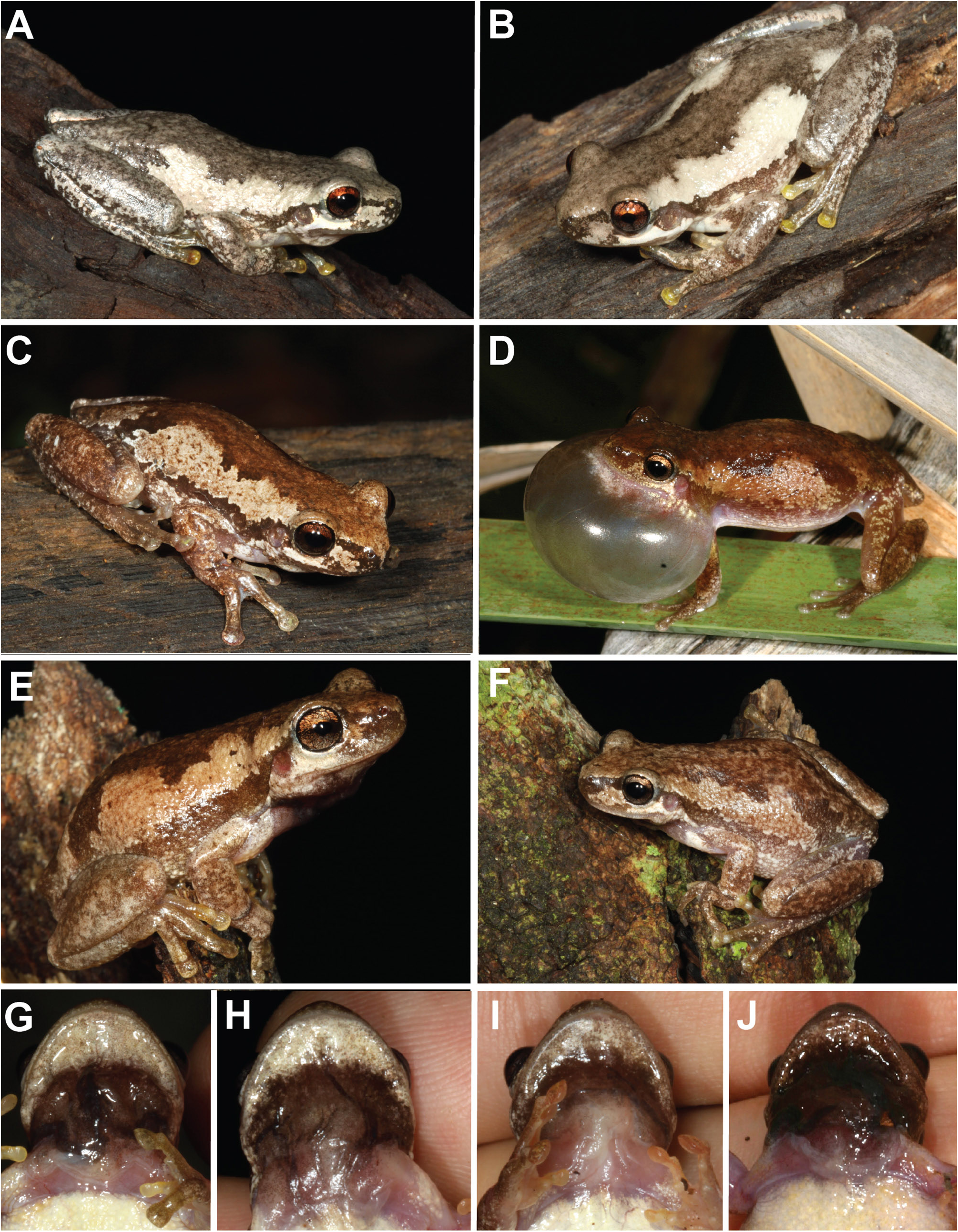
Variation in colour in life is described from images ( Fig. 12 View FIGURE 12 ). Dorsal colouration varies from cream (e.g. Fig. 12A View FIGURE 12 ) to a warm medium brown (e.g. Fig. 12D View FIGURE 12 ), with a distinct darker brown or brownish-grey patch across head and down the back, narrowing in width over the axilla and then also on the mid-dorsum. Some individuals mottled (e.g. Fig. 12F View FIGURE 12 ). Paler mid-dorsal line absent. Distinctly darker dorsolateral line running from snout, through eye, over tympanum, and down side of body to groin varies from medium- to dark- brown Bright white patch on upper lip between lower margin of eye and insertion of the arm. Dorsal surface of limbs medium-brown; finger and toe tips vary from medium-brown to pale yellow (e.g. Fig. 12A, B View FIGURE 12 ). Back of thighs transparent pinkish-orange or yellow-orange with varying amounts of darker brown pigment and opaque, creamy yellow flecks (e.g. Fig. 5A–C View FIGURE 5 ). Belly white; vocal sac in males black when deflated and grey when inflated ( Fig. 12G–J View FIGURE 12 ). Iris copper-brown (e.g. Fig. 12E, F View FIGURE 12 ) to reddish-copper (e.g. Fig. 12A View FIGURE 12 ).
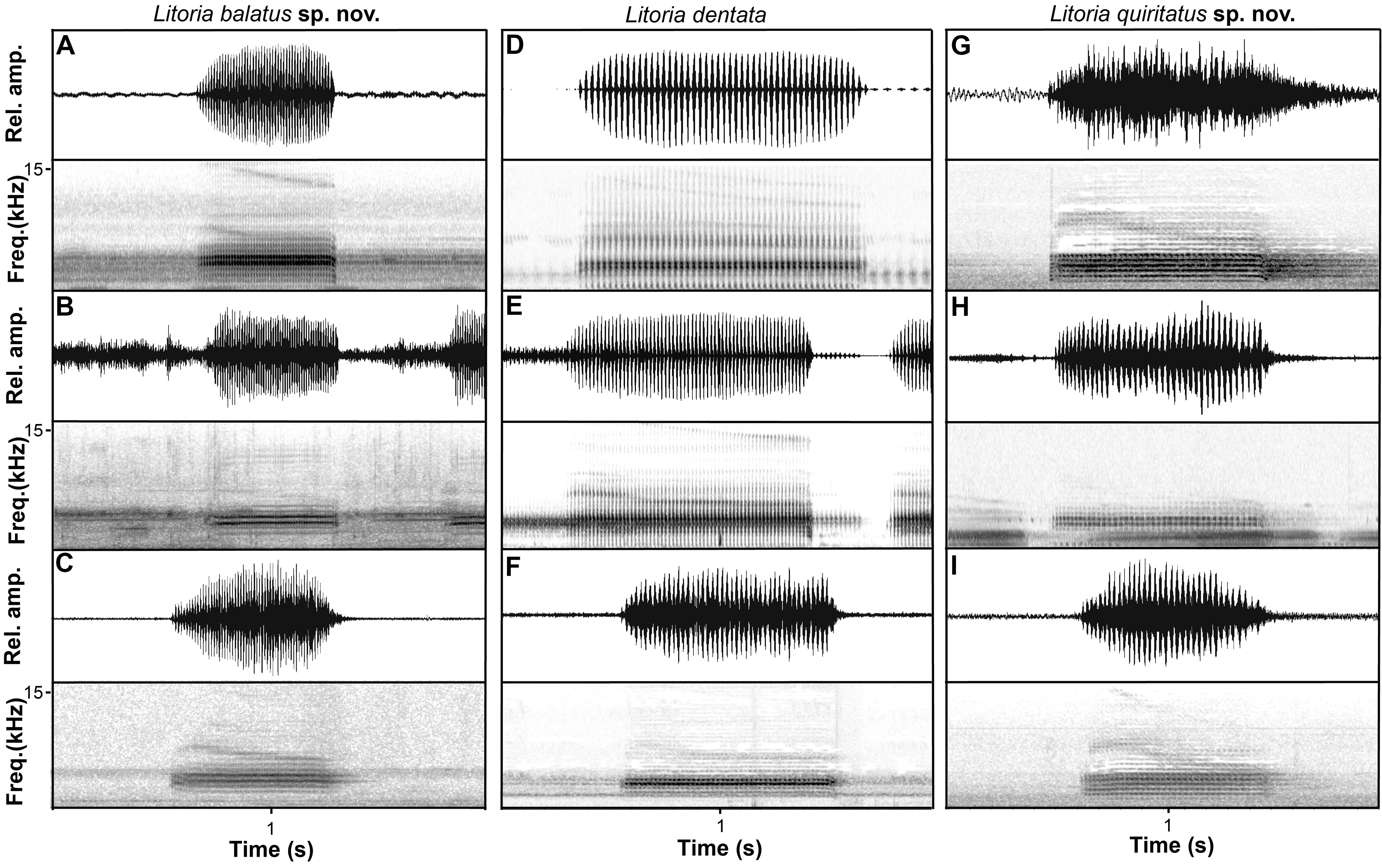
Advertisement call. Call descriptions are based on the calls of eight individuals, including the holotype ( Table 6 View TABLE 6 , Figs. 6 View FIGURE 6 , 8 View FIGURE 8 ). The advertisement calls of L. balatus sp. nov. comprises a single, highly-pulsed note. Individuals had a mean call duration of 0.63– 1.12 s and an average of 45–77 uniformly spaced pulses repeated at a rate of 57–89 pulses/s. Calls were amplitude modulated, increasing either smoothly or rapidly to a peak at approximately 10–20% of the call duration, but with much variation. The dominant frequency was 3.4–4.3 kHz.
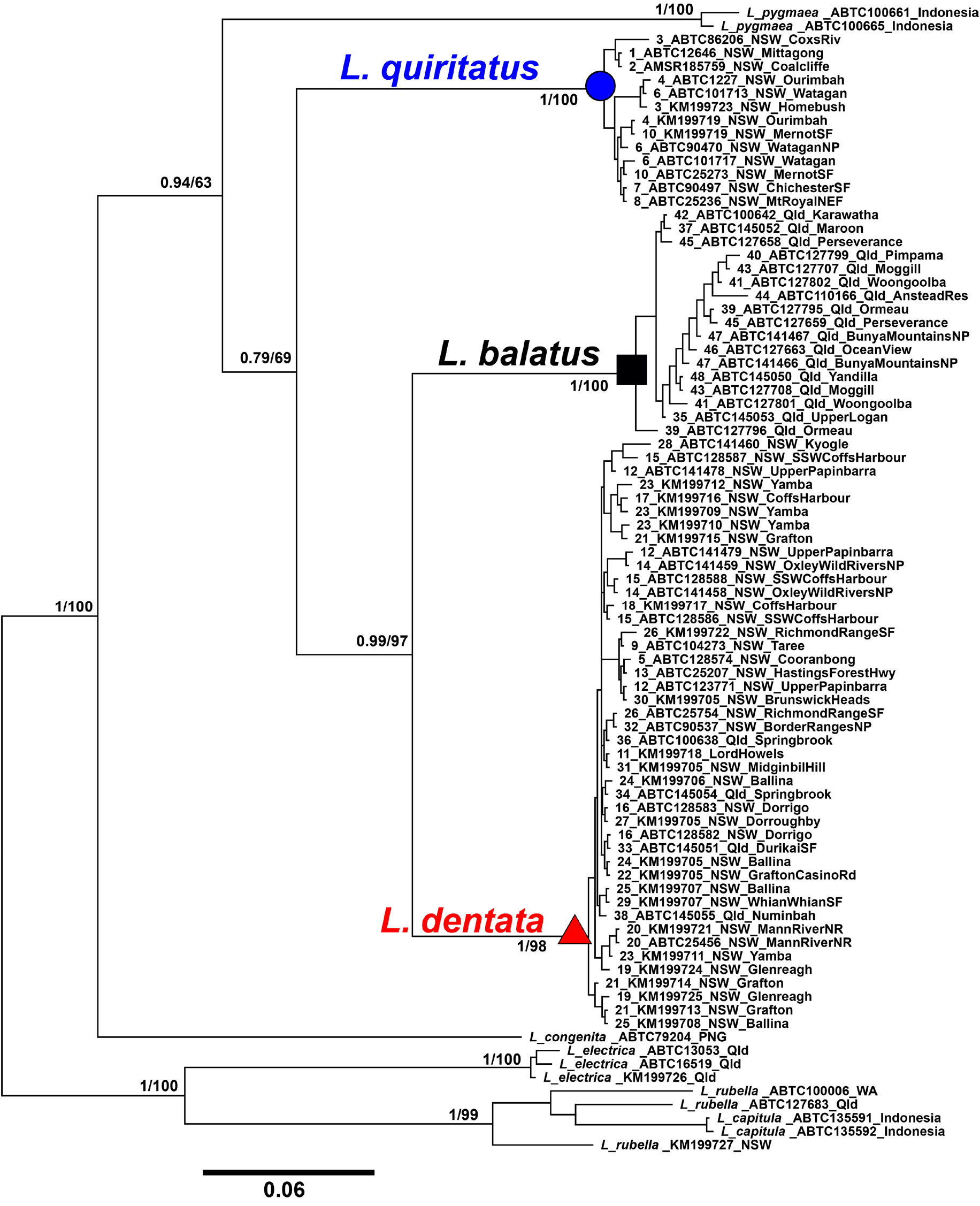
Comparison with other species. The distribution of L. balatus sp. nov. overlaps with L. rubella and may overlap with L. dentata in the Scenic Rim area of Queensland, but it is allopatric with the other six members of the L. rubella species group ( L. quiritatus from southern NSW and eastern Victoria, L. electrica from north-western Queensland, L. congenita and L. pygmaea in New Guinea and L. capitula on Tanimbar Islands, Indonesia). It can be distinguished from L. rubella by the presence of a continuous, irregularly edged, dark brown dorsal band and a less robust body (i.e. head much narrower than body in L. rubella ). In addition, the call of L. balatus sp. nov. is much higher pitched, of longer duration, with more pulses and a faster pulse repetition rate than that of L. rubella in south-eastern Queensland. Litoria balatus sp. nov. is widely sympatric with L. rubella , and with the two species often calling together these differences in advertisement call are readily apparent. Litoria balatus sp. nov. can be distinguished from L. electrica by the presence of a continuous, irregularly edged, dark brown dorsal band (versus two dark chocolate-coloured bars across the dorsum). It can be distinguished from the New Guinean species L. congenita and L. pygmaea by absence of light spots (versus large and conspicuous in L. pygmaea , smaller and more variable in L. congenita ) on dark dorsal background. It can be distinguished from L. capitula by the absence of distinctive pale markings above the groin, vent and along lower leg that are present in L. capitula . L. balatus sp. nov. can be distinguished from L. dentata by males having a vocal sac that is black when deflated and inflated (versus black or very dark yellowish black when deflated and yellowish brown when inflated). It can be distinguished from L. quiritatus sp. nov. by males having a vocal sac that is black (versus yellow when deflated and inflated). It can be distinguished further from L. dentata and L. quiritatus sp. nov. by having a distinct dorsolateral line continuing to groin (versus diffusing above insertion of arm), and more slender build ( Fig. 5 View FIGURE 5 ). From a genetic perspective, apomorphic nucleotide states at 28 sites in the mitochondrial ND4 gene reliably diagnose L. balatus sp. nov. from L. dentata and L. quiritatus sp. nov. ( Table 7 View TABLE 7 ).
Etymology. The specific epithet, balatus , is a masculine Latin 4 th declension noun, meaning “a bleating”, used as a noun in apposition to the genus name.
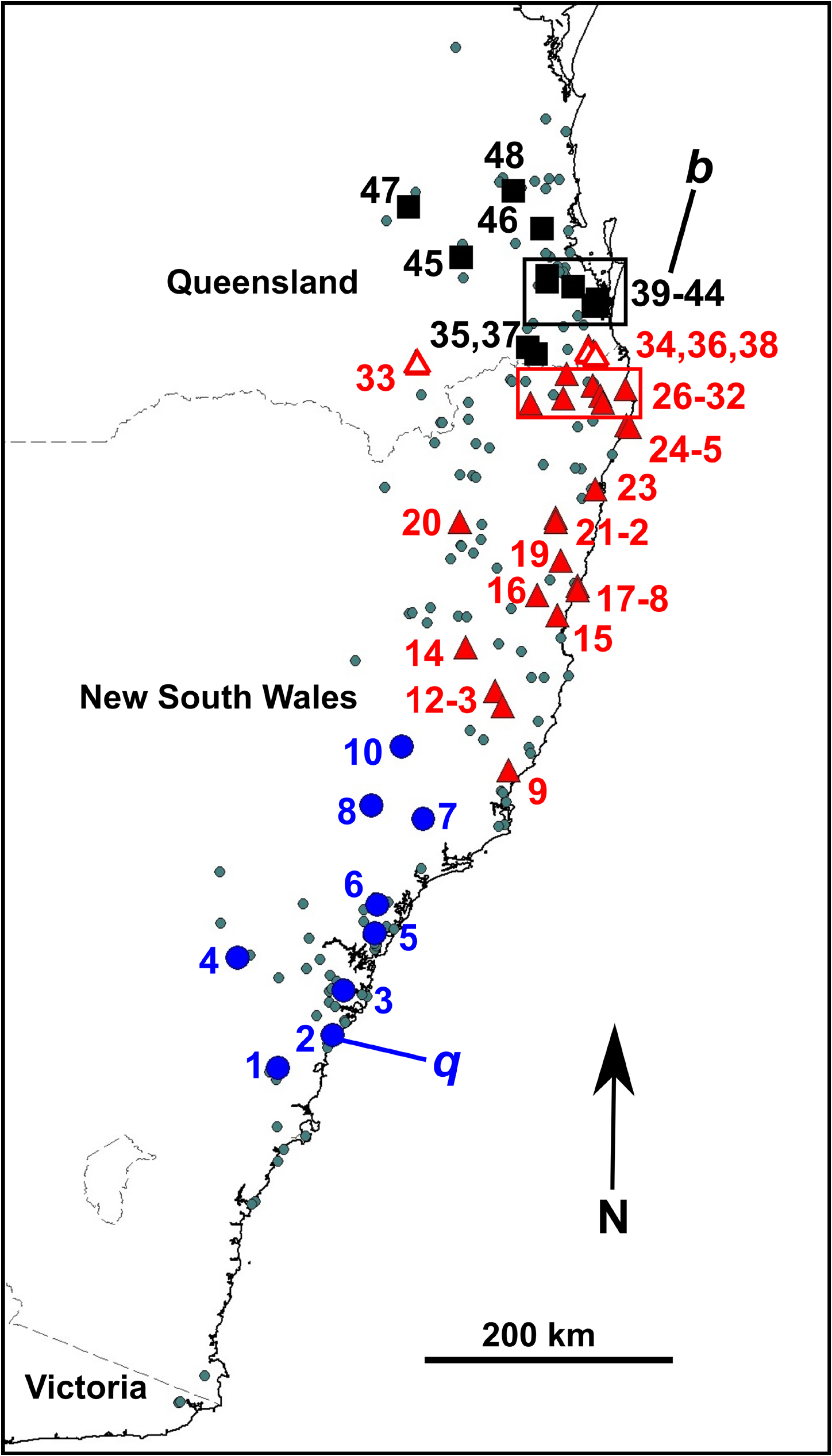
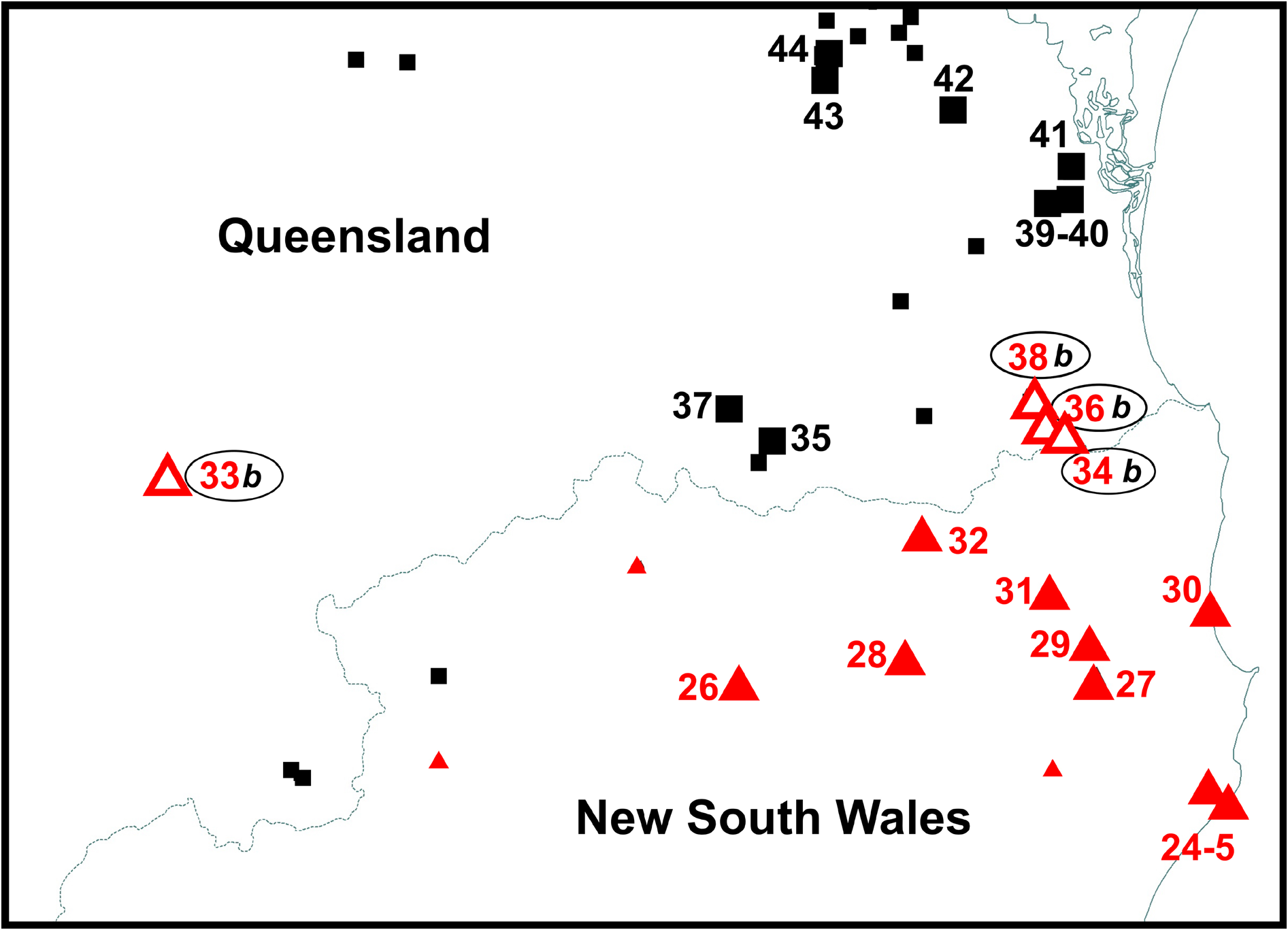
Distribution. Litoria balatus sp. nov. is known currently from south-eastern Queensland from the Maryborough district in the north (Biggenden, QM J24056 View Materials ) to the foothills of the scenic rim (Barney View, QM J96341 View Materials ), west to the Bunya Mountains. There are records of L. dentata sensu lato further north (e.g. Atlas of Living Australia), but there are no specimens or photographs available to verify these. The northernmost record of L. balatus sp. nov. in the FrogID project to date is in Maryborough. We are aware of a number of cases of Litoria rubella being misidentified as L. dentata sensu lato. Juvenile Litoria rubella , especially in the Gladstone region, often have extensive dark markings on the dorsum, causing confusion. We sequenced one such juvenile L. rubella (QM J90515 View Materials , ABTC 127683) from the Mount Larcom area, near Gladstone to confirm its identity ( Fig. 2 View FIGURE 2 ). Further surveys are required to ascertain the northern distributional limits of L. balatus sp. nov.
Litoria balatus sp. nov. has a patchy distribution and is apparently absent from wallum habitats and from larger expanses of rainforest ( Fig. 1 View FIGURE 1 ). It occurs from near sea level, e.g., around the Woongoolba area (QM J92771 View Materials , ABTC 127802) to ~ 600m in the Bunya Mountains and Conondale Range.
The ranges of Litoria balatus sp. nov. and L. dentata are in close proximity in the Scenic Rim on the NSW-Queensland border. We found individuals with discordant mtDNA ancestry and phenotypes (body habitus) in this area ( Fig. 7 View FIGURE 7 ) for which we currently do not have nuclear genotype data to establish their species identity. The Scenic Rim is characterised by mountainous terrain rising steeply above the coast and floodplains. Consequently, rainfall, temperature and vegetation vary markedly over very short distances. These patterns strongly influence the distribution of many vertebrate species in this region, and it is recognised as an area of overlap between northern and southern faunal groups ( Bryant & Krosch 2016). Within Anura , for example, Pseudophryne coriacea occurs in the Scenic Rim area but is replaced to the north by P. raveni ( Ingram & Corben 1994) . The Scenic Rim area is also the northern limit for the anuran genera Philoria and Australian Lechriodus (the latter also has species in New Guinea). Additional nuclear genotyping is required in the Scenic Rim area to better understand the distribution of the two Litoria species and in particular to assign species identity to records of this species complex from the Springbrook-Numinbah Valley, the O’Reilly’s Plateau-Christmas Creek-Canungra area or from the Great Dividing Range from Girraween National Park, through Main Range National Park to Toowoomba, and from Durikai State Forest.
Ecology. Most publications concerning L. dentata sensu lato do not include observations or data from Queensland, hence existing information on L. balatus sp. nov. is scant. Delvinquier (1986) recorded the protozoan parasite Myxidium immersum from Samford, Queensland, in a host now attributable to L. balatus sp. nov. Murray et al. (2007) list a single histological specimen of L. dentata from Belli Creek SEQ that was negative for the fungal pathogen Batrachochytrium dendrobatidis : this record is now attributable to L. balatus sp. nov. The following information is from field observations (H. B. Hines & E. Meyer unpubl. data) or from FrogID as acknowledged.
Litoria balatus sp. nov. is a species of open forests, woodlands and occasionally rainforest. It appears to be largely absent from the coastal wallum communities and has not been recorded from any of the large sand islands of south-east Queensland ( Hines & Meyer 2011). Data from FrogID (>630 records from 10 November 2017 – 30 June 2021) show that this species frequently calls from disturbed areas, with 14% of records in urban habitats and 60% in rural areas.
The species has been recorded calling from September to March via the FrogID project. Males call occasionally from treetops and buildings during the day, particularly in hot and humid weather but persistent calling and congregations of calling males typically occur at night following heavy or prolonged rainfall during the warmer months. Amplexus has been observed in all months from October to March. Breeding occurs in shallow ephemeral drainage lines or wetlands with emergent vegetation, with small dams also regularly used. Oviposition, eggs and larval morphology, development and behaviour have not been documented.
Conservation status. Litoria balatus sp. nov. is the most restricted of the three species, with an estimated Extent of Occurrence of 73,000 km 2. There were no ill or dead specimens of L. balatus sp. nov. in the study of Berger et al. (2004) that examined a large number of diseased wild frogs from south-eastern Queensland. The fungal pathogen Batrachochytrium dendrobatidis has not been recorded in L. balatus sp. nov. (Murray et al. 2007) although screening is very limited (n=1). There are no documented or suspected population declines. Given the large Extent of Occurrence, persistence in disturbed areas and a lack of evidence of population declines, the species likely meets IUCN Red List criteria ( IUCN 2012) for Least Concern.
| QM |
Queensland Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.