Magelona tinae Nateewathana & Hylleberg, 1991
|
publication ID |
https://doi.org/ 10.5281/zenodo.198268 |
|
DOI |
https://doi.org/10.5281/zenodo.6208092 |
|
persistent identifier |
https://treatment.plazi.org/id/03BDDD14-E47C-5539-FF41-F980BE27F929 |
|
treatment provided by |
Plazi |
|
scientific name |
Magelona tinae Nateewathana & Hylleberg, 1991 |
| status |
|
Magelona tinae Nateewathana & Hylleberg, 1991 View in CoL
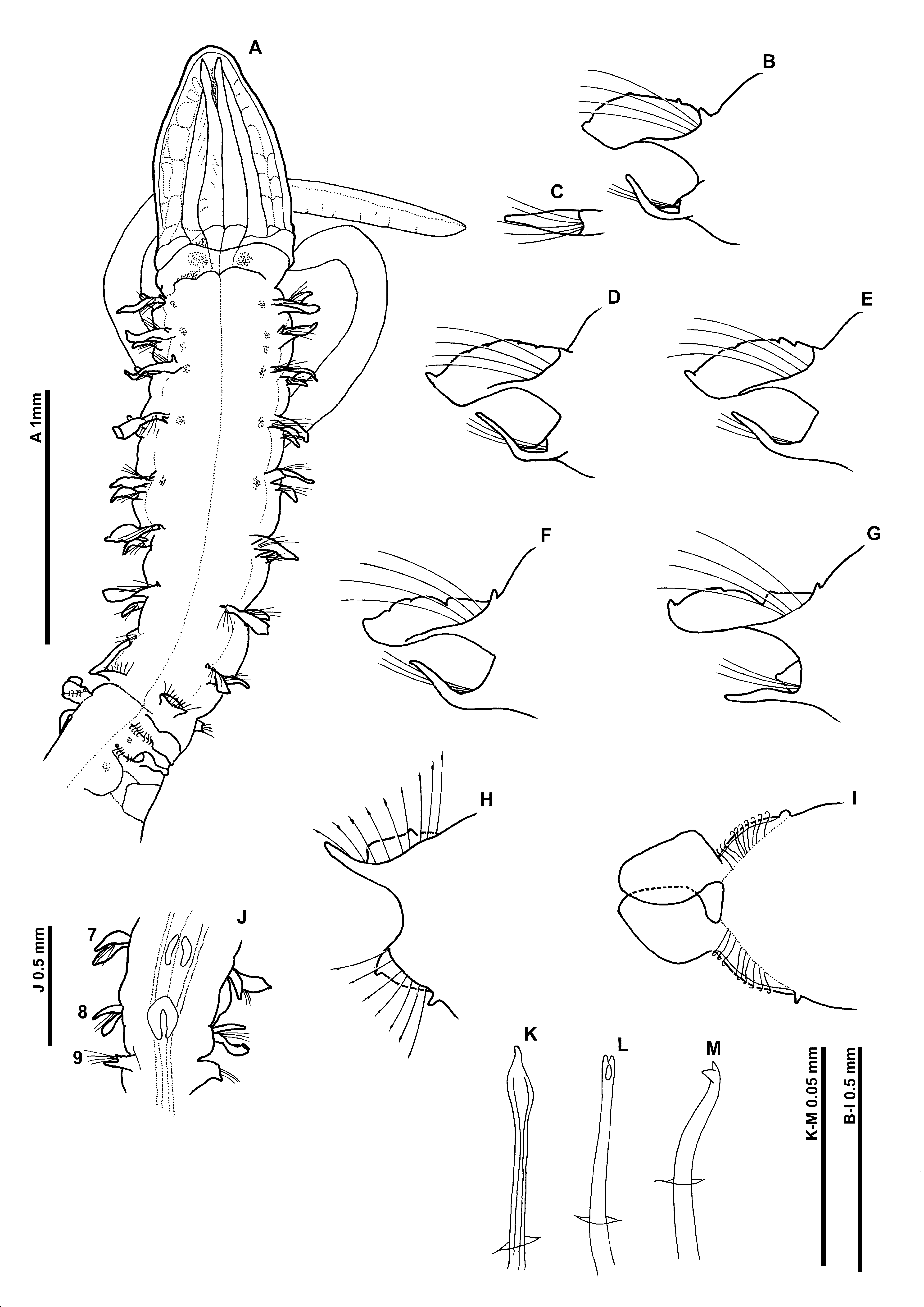
Figure 11 View FIGURE 11
Magelona tinae Nateewathana & Hylleberg, 1991: 181 View in CoL –183, fig. 10.
Material examined. Phuket Island, Thailand—Kamala Bay, fine sand, 10 m, Paratype ( PMBC 3180; c), 17th September 1980; Sta. IV–3 6/3 Bang Tao Bay, 10 m ( PMBC 4251; af), 9th November 1982; Sta. IV–3 3/1 Bang Tao Bay, 10 m ( PMBC 4253; af), 9th November 1982; Sta. IV–3 6/1 Bang Tao Bay, 10 m ( PMBC 4254), 9th November 1982. Holotype not available for examination.
Diagnosis. Prostomium longer than wide, slender triangular, without prostomial horns. Notopodia of chaetigers 1–8 with subrectangular postchaetal lamellae, upper edges lightly crenulated, bilobed on several posterior chaetigers. Short cirriform dorsal processes present on chaetigers 1–8. Neuropodia with single long slender processes in a ventral position of similar size throughout thorax. Neuropodia of chaetiger 8 with digitiform, prechaetal processes, in addition to subtriangular postchaetal lamellae. Chaetiger 9 with low postchaetal lamellae, small neuropodial processes present ventrally at inner margins of chaetal rows. Chaetigers 1–8 with capillary chaetae, chaetiger 9 with mucronate chaetae. Abdominal lateral lamellae spatulate, bluntly pointed, basally constricted. Hooded hooks tridentate, in two groups, vis–à–vis. Anteriorly and posteriorly open pouches present on abdominal chaetigers.
Description. A slender species ( Fig. 11 View FIGURE 11 A); abdomen thicker than thorax. Paratype complete in two fragments, (25 & 42 chaetigers) prostomium 1.0 mm long, 0.6 mm wide; thorax (including prostomium) 2.6 mm long, 0.6 mm wide at widest point; abdomen 0.5 mm wide; total length 16.6 mm for 67 chaetigers (note: recorded as 65 chaetigers by Nateewathana & Hylleberg 1991). Other material 4.7 mm – 14.5 mm for 25–49 chaetigers.
Prostomium elongate, much longer than wide (L:W ratio 1.67), slender triangular, without prostomial horns, anterior margin smooth, rounded, formed into blunt tip; eyes absent. Two pairs of prominent longitudinal dorsal muscular ridges, outer pair (slightly shorter) abutting inners for entire length, inner pair reaching the distal tip of prostomium, where they diverge only very slightly. Fairly distinct quadrangular areas either side of muscular ridges. Proboscis not everted in any observed specimen. Palps arising ventrolaterally from base of prostomium, reaching around chaetigers 20–25, non–papillated region reaching chaetigers 3–4. Papillae relatively long. Proximally with 2 rows of papillae either side of inconspicuous ventral groove, distally 1–2 rows. Both palps retained on two specimens (PMBC 4251 & 4253), right–hand one only on paratype (distal tip broken).
Peristomium achaetous, of similar size to chaetiger 1. Chaetigers 1–7 similar; parapodia biramous with triangular notopodial prechaetal lamellae confluent with subrectangular postchaetal lamellae, distal tips pointed. Postchaetal lamellae increasing in size gradually long thorax ( Figs 11 View FIGURE 11 B–F). Upper edges of postchaetal lamellae very lightly crenulated, degree of crenulation variable; an indistinct notch present in the upper margins of the sixth chaetiger ( Fig. 11 View FIGURE 11 E) which increases in size and develops into bilobed lamellae from chaetiger 7 ( Fig. 11 View FIGURE 11 F). Single short, slender, cirriform prechaetal superior process (DML) present on chaetigers 1–8, of similar size throughout thorax.
Neuropodia of 1–7 with single long slender cirriform processes ventrally (VNL), directly under chaetae but becoming slightly prechaetal by chaetiger 5, distal tips pointed, of similar size throughout thorax. Pre– and postchaetal lamellae well–developed, encircling chaetae cuff–like ( Fig. 11 View FIGURE 11 C).
Chaetiger 8 ( Fig. 11 View FIGURE 11 G): Notopodial lamellae bilobed, dorsalmost portion triangular; lateral portion more rounded, upper edge minutely crenulate, with pointed tip. Neuropodial processes (VNL) prechaetal, digitiform, marginally shorter and thinner than on preceding chaetigers. Low prechaetal lamellae confluent with subtriangular postchaetal lamellae, encircling chaetae superiorly, cuff–like ( Fig. 11 View FIGURE 11 G). Chaetae of chaetigers 1–8 simple winged capillaries.
Chaetiger 9: shorter than preceding chaetigers ( Fig. 11 View FIGURE 11 A). Notopodial prechaetal lamellae low, confluent with higher postchaetal lamellae, upper edges minutely crenulate ( Fig. 11 View FIGURE 11 H). Lamellae encircling chaetae underneath as slender cirriform lateral expansions. No superior processes (DML) observed. Neuropodia similar to notopodia, without lateral expansions, small ventral prechaetal processes present (VML of some authors). Chaetae spatulate ( Fig. 11 View FIGURE 11 K), arranged in arcs, chaetae longer towards margins of each fan.
Oval markings present ventrally, level with lamellae of chaetiger 8 ( Fig. 11 View FIGURE 11 J), and two reniform shaped markings present level with chaetiger 7.
Abdominal chaetigers ( Fig. 11 View FIGURE 11 I) with broad, spatulate lateral lamellae, of about equal size in both rami, basally constricted, bluntly pointed, overlapping in anterior abdomen. Lamellar shape shows some variation. Extension of lateral lamellae behind chaetal rows present but not well–developed. Small triangular processes (DML & VML) present at inner margins of chaetal rows. Lamellae decreasing in size along abdomen, becoming more slender.
Abdominal chaetae ( Figs 11 View FIGURE 11 L, M) tridentate hooded hooks of similar size, superior two fangs parallel, above main fang. Approximately 10 hooks per rami, hooks in two groups, main fangs vis–à–vis, group at inner margins of chaetal rows with around twice number of chaetae ( Fig. 11 View FIGURE 11 I). Paired anteriorly open pouches between chaetigers 11 and 12 (Σ configuration of Fiege et al. 2000 —see discussion), well–developed, large membrane, often extruded, bounded between two cuticular flaps. Unpaired posteriorly open pouches (C configuration of Fiege et al. 2000 —see discussion) present posteriorly, on alternate chaetigers, more or less regularly. Pouches quite large, often convoluted. On paratype, pouches observed on 11, 22L, 24R, 26L, 28R, 30L, 32R, 34L, 36R, 38L, 40R, 42L, 44R, 46L, 48R, 50L, 52R, 54L, 56R, 58L, 60R, 62L and 64R. Pygidium with two short digitiform anal cirri, left–hand cirri shorter than right (presumed damaged?).
Colour. Specimens generally pale, with cream to white coloration. Staining with methyl green shows no clear pattern, but an overall diffuse stain. Small speckled areas dorsally, next to parapodia on chaetigers 1 to 5 are present ( Fig. 11 View FIGURE 11 A). Slight staining evident abdominally between parapodia. Nateewathana & Hylleberg (1991) recorded a diffuse dorsolateral staining pattern with methyl blue between chaetigers 1 and 9, and blue staining ventrally between chaetigers 6 and 9.
Habitat. Type specimens found in very fine–fine sand, 10 m.
Remarks. Several differences from the original description of M. tinae have been noted; the shape of abdominal lateral lamellae, being more rounded/spatulate than figured ( Nateewathana & Hylleberg 1991, fig. 10F), and the slightly crenulate nature of the upper edges of the notopodial postchaetal lamellae of chaetiger 9. Foliaceous posteriorly open pouches are present in addition to the paired pouches between chaetiger 11–12 as reported by Nateewathana & Hylleberg (1991).
Morphologically, M. tinae is extremely similar to M. obockensis in terms of prostomial shape, form of thoracic and abdominal lamellae and presence of mucronate chaetae on chaetiger 9. Many similarities in the shape of the thoracic postchaetal lamellae exist; presence of pointed tips on lateral margins, minutely crenulated upper edges, and the presence of bilobed lamellae in the posterior thorax. However, slight variations between the two species have been observed. Magelona tinae differs from M. obockensis in terms of thoracic notopodial lamellar shape being more rounded oblong and less foliaceous; the postchaetal lamellae of chaetiger 9 less crenulated; prechaetal superior processes (DML) on chaetiger 9 were not observed; bilobed thoracic postchaetal lamellae being present only on chaetigers 7–8; postchaetal expansions of the lateral lamellae in the abdomen less developed.
Small indistinct markings (not swollen) resembling the shape of those ventral swellings of M. obockensis were seen on both type and non– type material of M. tinae , level with chaetigers 7–8. Nateewathana & Hylleberg (1991, fig. 10H) also drew several structures on the venter of the holotype between chaetigers 6–8 (although they made no mention of this in their description). Nateewathana & Hylleberg (1991) noted staining ventrally between chaetigers 6 and 9 with methyl blue, this appears to be associated with these structures in their figured specimen. The staining patterns with methyl green for both M. tinae and M. obockensis are very similar, however, no white speckling associated with the venter was observed in M. tinae .
Some of the variations seen between these two species could be explained by size, M. tinae being somewhat smaller than either M. obockensis or M. heteropoda . The development of the bilobed and crenulated notopodial lamellae, and ventral swellings for instance may be linked directly to size. The lack of dorsal superior process on chaetiger 9 in M. tinae may also be linked to size. Those of M. obockensis were small and difficult to see even on larger specimens, and may be perceived to be lacking in smaller specimens. It is clear that these species are extremely morphological similar, further investigation between specimens of a similar size is warranted, in order to investigate these variations, before any conclusions can be made about the status M. tinae .
| PMBC |
Phuket Marine Biological Centre |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Magelona tinae Nateewathana & Hylleberg, 1991
| Mortimer, Kate 2010 |
Magelona tinae
| Nateewathana 1991: 181 |