Plesionika reflexa Chace, 1985
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4382.3.9 |
|
publication LSID |
lsid:zoobank.org:pub:4F3ADFAA-6CA0-4305-9ED9-5A35F9873B25 |
|
DOI |
https://doi.org/10.5281/zenodo.5980999 |
|
persistent identifier |
https://treatment.plazi.org/id/038387BB-F813-A53D-37D0-FDE4FC752CD2 |
|
treatment provided by |
Plazi |
|
scientific name |
Plesionika reflexa Chace, 1985 |
| status |
|
Plesionika reflexa Chace, 1985 View in CoL
( Figs. 2 View FIGURE 2 , 3 View FIGURE 3 )
Pandalus View in CoL ? ensis View in CoL — Alcock & Anderson, 1899: 284. (non A. Milne-Edwards, 1881).
Pandalus (Plesionika) ensis View in CoL — Alcock 1901: 96. (non A. Milne-Edwards, 1881).
Plesionika ensis View in CoL — Suseelan & Mohamed 1969: 88, fig. 1. (non A. Milne-Edwards, 1881).
Plesionika reflexa Chace, 1985: 108 View in CoL , fig. 49. (type locality: Philippines); Hayashi 1986: 137, fig. 88; Hanamura & Takeda 1987: 116; Kensley et al. 1987: 318; Takeda & Hanamura 1994: 23, fig. 10; Chan & Crosnier 1997: 194, figs. 3, 24; Fransen 2006: 77, fig. 23; Li 2006: 1290; De Grave & Fransen 2011: 450.
Material examined. Sakthikulangara fishing port, Kollam district , Kerala, 200–300 m 23 Nov 2015, 1 ovig. female cl 15.0 mm ( CMFRI) ; 250 m, 27 April 2017, 1 male cl 14.3 mm (CMFRI ED.2.4.3.8), 1 male cl 14.6 mm, 1 ovig. female cl 18.9 mm, 1 specimen sex undetermined cl 12.8 mm (NTOU M02081 View Materials ).
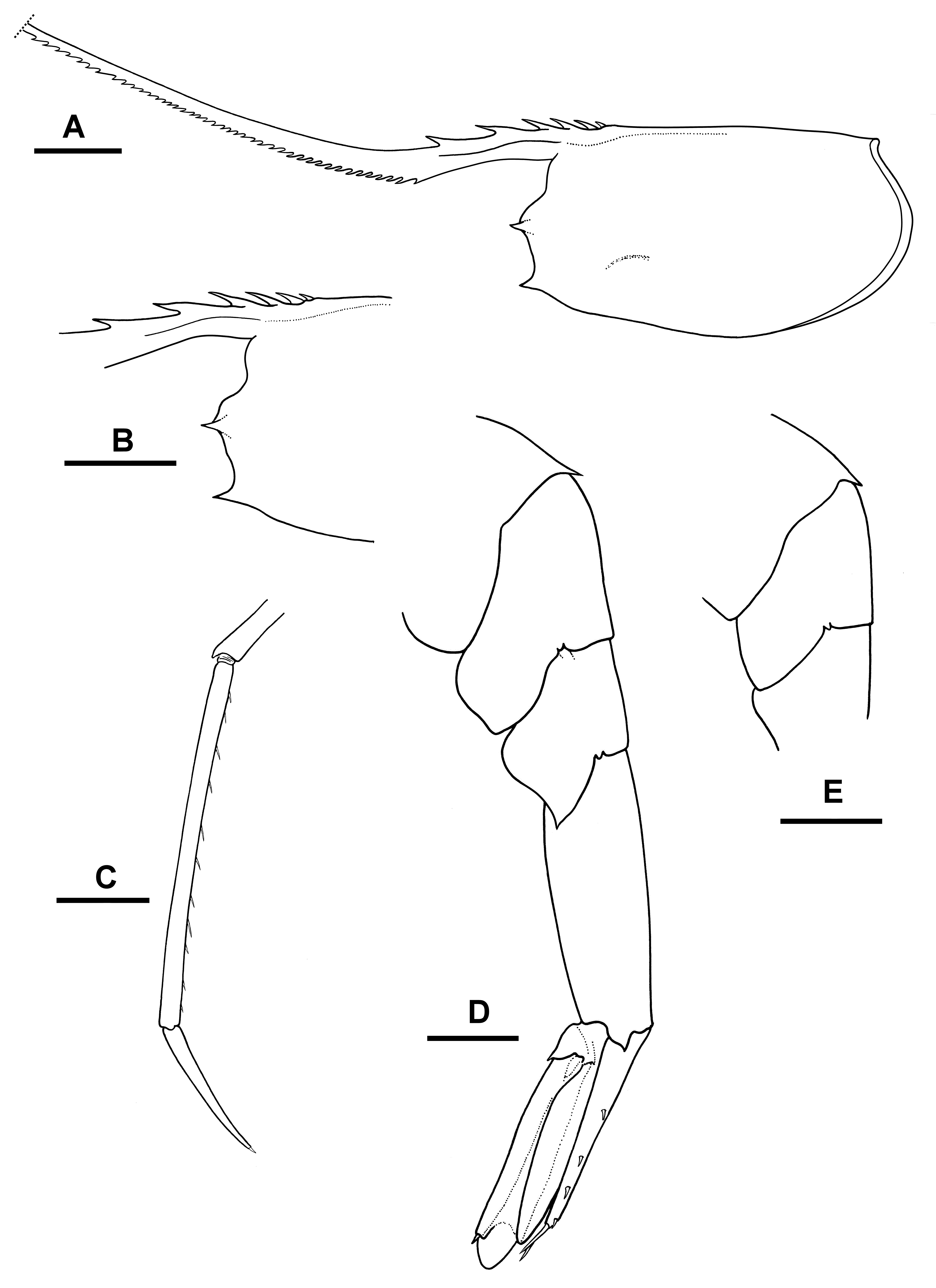
Diagnosis. Rostrum long, about 2 times longer than carapace length and far overreaching scaphocerite, slightly curved downwards at basal part but slightly recurved upwards and nearly straight after passing middle of antennular peduncle; dorsal margin armed with 6 teeth at basal part as well as 1 subapical tooth, none with barbed tip, basal teeth restricted behind distal end of antennular peduncle and not forming crest, with 2–3 of them posterior to orbital margin and posterior 2–4 teeth bearing incomplete basal suture; ventral margin with 40–43 teeth. Postrostral ridge distinct but blunt, extending to about middle of carapace and not highly elevated. Carapace only with short, fainted hepatic grooves; antennal and pterygostomian spines similar and moderately large; orbital margin with upper part nearly vertically convex, lower part also convex. Eye with distinct ocellus. Stylocerite obtuse but distally pointed, laterally slightly folded upwards, just overreaching basal segment of antennular peduncle. Scaphocerite slender, 4.6–5.0 times as long as maximum width, with distolateral tooth just exceeding distal margin of lamina, basicerite spine long and markedly overreaching proximal end of lateral margin of scaphocerite. Maxilliped III bearing well developed epipods, penultimate segment slightly shorter than terminal segment. Only anterior 3 pereiopods with epipods, those at pereiopods I and II well developed, epipod at pereiopod III small and delicate. Pereiopod II subequal and bearing 15–19 carpal articles. Pereiopod III exceeding scaphocerite by length of dactylus and a short portion of propodus, dactylus 0.41–0.47 times as long as propodus, accessory distal spine minute and little separated from base of terminal spine. Abdomen with tergite III bearing well-developed poteromesial spine, which more or less recurved upwards; pleuron IV posteroventrally rounded while pleuron V bearing distinct posteroventral denticle; somite VI 2.5–2.9 times longer than maximum height; telson 0.8 times as long as somite VI and bearing 3 pairs of dorsolateral spines (excluding pair adjacent to posterior margin of telson).
Distribution. Widely distributed in the Indo-West Pacific from Gulf of Aden to Japan and French Polynesia, at depths of 191– 910 m.
Remarks. The “Diagnosis” given above was based on the present four Indian specimens; with only one of them (male cl 14. 8 mm) having intact rostrum and another one (male cl 14.3 mm) without any complete pereiopod III. Plesionka reflexa belongs to the “ P. ensis ” group, which currently includes only two recognized species ( Chan & Crosnier 1997). Species of the “ P. ensis ” group are very characteristic in having a distinct posteromesial spine on the abdominal tergite III, rostrum long but with only a few dorsal teeth restricted to base and bearing numerous ventral teeth, posterior pereiopods rather robust and not particularly long. The two species in this group are assumed to have discrete distribution, with P. ensis occurring in the Atlantic while P. reflexa is distributed in the Indo-West Pacific. The two species differ only in P. ensis having the posteromesial spine on the abdominal somite III straight and the pereiopod III with shorter dactylus. On the other hand, P. reflexa has the abdominal somite III posteromesial spine more or less recurved upwards and longer dactylus at the pereiopod III (see Chace 1985). However, as noticed by many workers (see Hanamura & Takeda 1987; Chan & Crosnier 1997; Fransen 2006), Indo-West Pacific material of this group has large variations in the curvature of the abdominal posteromesial spine from very straight to distinctly recurved though this spine is always straight in Atlantic specimens. The proportional length of the dactylus of the pereiopod III is also highly variable (from 0.09 to 0.46 as long as propodus) at various localities in both the Atlantic and Indo-West Pacific (see Chace 1985; Chan & Crosnier 1997; Fransen 2006). Nevertheless, the pereiopod III dactylus is shorter (0.11–0.25 as long as propodus) in the topotypic material of P. ensis in the western Atlantic (type locality: near Barbados) but longer (0.30–0.46 as long as propodus) in the topotypic material of P. reflexa in the Philippines. The present Indian specimens have the abdominal posteromesial spine from nearly straight ( Fig. 2E View FIGURE 2 ) to distinctly recurved ( Fig. 2D View FIGURE 2 ), and even slightly longer pereiopod III dactylus ( Fig. 2C View FIGURE 2 , 0.41–0.47 as long as propodus).
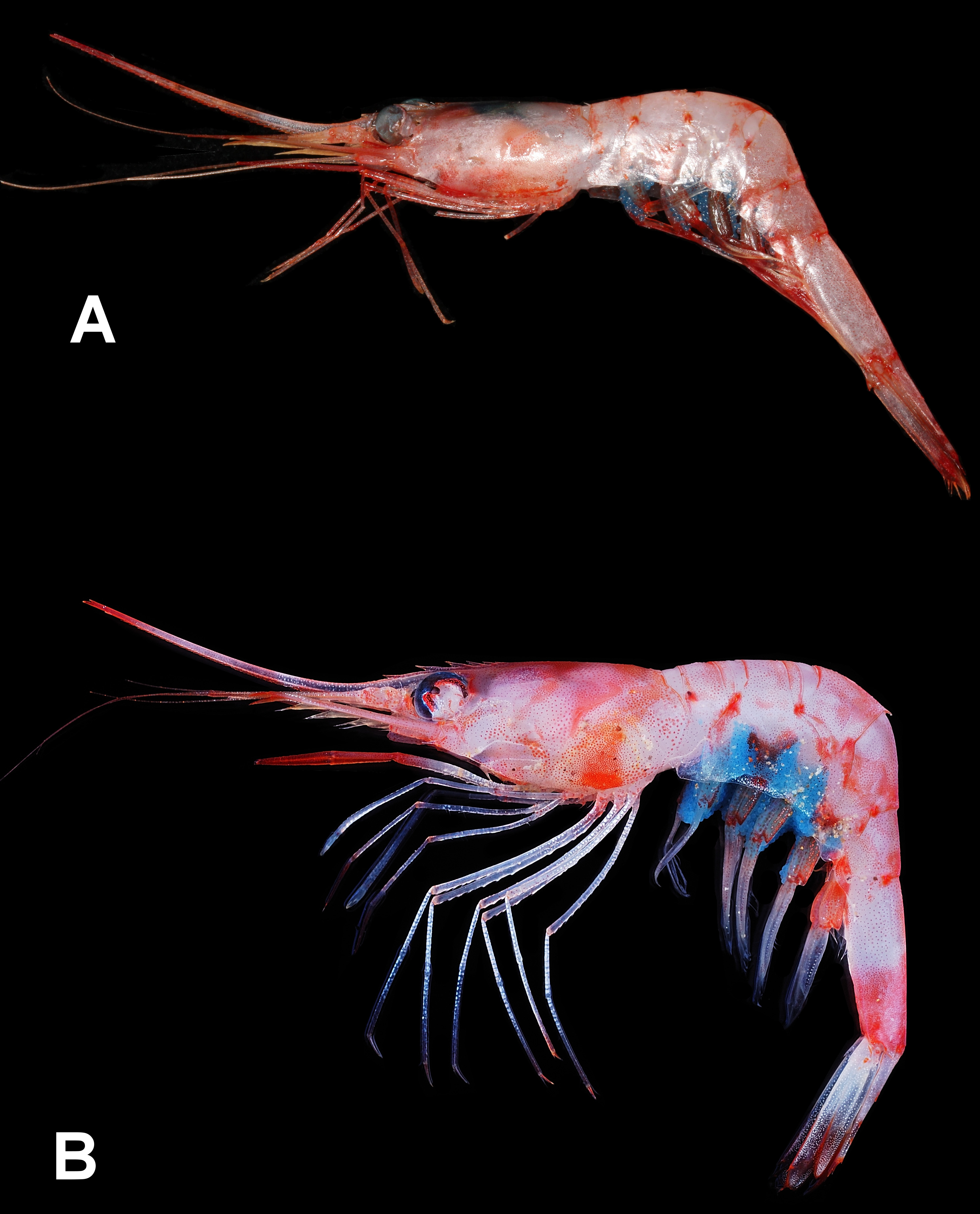
As discussed in Chan & Crosnier (1997), the large variations in the curvature of the abdominal posteromesial spine, length of pereiopod III dactylus and rostrum in the material of P. ensis and P. reflexa may indicate that there are actually many more species in the “ P. ensis ” group similar to the situation in the “ P. martia ( A. Milne-Edwards, 1883) ” and “ P. narval ( Fabricius, 1787) ” groups ( Chace 1985; Chan & Crosnier 1991). The present Indian material further differs in the epipods at the pereiopods III and IV more reduced or even absent. The present specimens have the epipods of the pereiopods III half the size of those at preceding pereiopods and rather delicate. Moreover, the epipod is completely absent at the pereiopod IV in all specimens. The presence or absence of epipods at the pereiopods has often been considered as of specific value in Plesionika ( Chace 1985; Li & Komai 2003). Fransen (2006) also reported that P. reflexa material in the western Arabian Sea and Burma from northern Indian Ocean differs from the typical form in having the epipods at the pereiopods III and IV reduced though a minute epipod is still present at the pereiopod IV. However, Suseelan & Mohamed (1968) mentioned that their SW Indian specimens carry well developed epipods at the anterior four pereiopods, and their fig. 1a showed that the dactylus of pereiopod III is about one fifth as long as the propodus. Genetic comparisons of the present Indian specimens (2 males, 1 ovig. female, CMFRI ED.2.4.3.8, NTOU M02081 View Materials ; GenBank nos. MG729438 View Materials -729440) with topotypic material of P. ensis ( Fig. 3A View FIGURE 3 from the Lesser Antilles: Guadeloupe, KARUBENTHOS 2015 stn DW 4609, MNHN IU-2013-19054, GenBank no. MG729442 View Materials , Poupin & Corbari 2016: fig. 6e, versus type locality Barbados) and P. reflexa ( Fig. 3B View FIGURE 3 from the Philippines: eastern Luzon, AURORA stn CP2695, NTOU M02080 View Materials , GenBank no. MG729441 View Materials , versus type locality S.E. Luzon) showed that there are high divergence in the barcoding gene COI amongst the specimens from different localities (9.3%, 10.3–10.7%, 14.2–14.5% divergences between material from Lesser Antilles/ Philippines, Philippines / India and Lesser Antilles/ India, respectively). However, there are only 0.0–0.3% genetic divergence amongst the Indian material including those with the abdominal posteromesial spine variably recurved ( Fig. 2D, E View FIGURE 2 ). The high genetic difference of the Indian form from topotypic material of both P. ensis and P. reflexa , and the reduction or absence of epipods at the pereiopod III and IV may urge the separation of the Indian form as another species. As pointed out by Chakraborty et al. (2015), however, species delimitation using COI data alone is still rather controversial for Plesionika . For example, the COI sequence divergences amongst seven Plesionika species from Northeast Atlantic and Mediterranean ranged from 2.5% to 18.3% (Matzen da Saliva et al. 2013) while that of P. quasigrandis Chace, 1985 materials from India and the Philippines can be as high as 5.8–8.4% ( Chakraborty et al. 2015). In views that P. ensis / reflexa have a very wide geographical range with highly variable morphological characters ( Chan & Crosnier 1997; Fransen 2006) but no coloration difference ( Fig. 3A, B View FIGURE 3 as compared to Kuberan et al. in press: fig. 1 and the color description of the Indian material provided by Suseelan & Mohamed 1968); and the recent finding of the development of the pereiopodal epipods can be rather variable in certain species of Plesionika (see Chan 2016), it may be more appropriate to erect further new species in the “ P. ensis ” group after a full revision on the group. For example, the recent review by Ahamed et al. (2017: tables 1, 2 and key) on Asian species of Plesionika included both P. ensis and P. reflexa . However, P. ensis was omitted in their systemic account. It is likely that all previous reports of P. ensis from India (e.g. Alcock & Anderson, 1899; Alcock, 1901; George 1966; Suseelan & Mohamed 1968; Suseelan 1974; Kurup et al. 2008; Rajool Shani s et al. 2012; Radhakrishnan et al. 2012; Samuel et al. 2016) represented the present form. Examination of more specimens from India, as well as the other parts of the Indian Ocean, will be necessary to fully understand the development of pereiopodal epipods and the variations in the length of the dactyli of the posterior pereiopods in the Indian Ocean material.
| CMFRI |
See FMRI |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Plesionika reflexa Chace, 1985
| Chan, Tin-Yam, Chakraborty, Rekha Devi, Purushothaman, P., Kuberan, G. & Yang, Chien-Hui 2018 |
Pandalus
| Alcock & Anderson, 1899 : 284 |
Pandalus (Plesionika) ensis
| Alcock 1901 : 96 |
Plesionika ensis
| Suseelan & Mohamed 1969 : 88 |
Plesionika reflexa
| Chace, 1985 : 108 |
| Hayashi 1986 : 137 |
| Hanamura & Takeda 1987 : 116 |
| Takeda & Hanamura 1994 : 23 |
| Chan & Crosnier 1997 : 194 |
| Fransen 2006 : 77 |
| Li 2006 : 1290 |
| De Grave & Fransen 2011 : 450 |