Tomopaguroides Balss, 1912
|
publication ID |
https://doi.org/ 10.5281/zenodo.5396681 |
|
persistent identifier |
https://treatment.plazi.org/id/B76D7017-FFD7-D86C-8D8F-FED4CCC2F9A1 |
|
treatment provided by |
Marcus |
|
scientific name |
Tomopaguroides Balss, 1912 |
| status |
|
Genus Tomopaguroides Balss, 1912 View in CoL
Tomopaguroides Balss, 1912: 104 View in CoL . — Gordan 1956: 342 (list). — McLaughlin 2003a: 128.
TYPE AND ONLY SPECIES. — Parapagurus valdiviae Balss, 1911 , by original designation.
DISTRIBUTION. — Indian Ocean off Somalia, Philippine, Salomon and Fiji islands.
DESCRIPTION
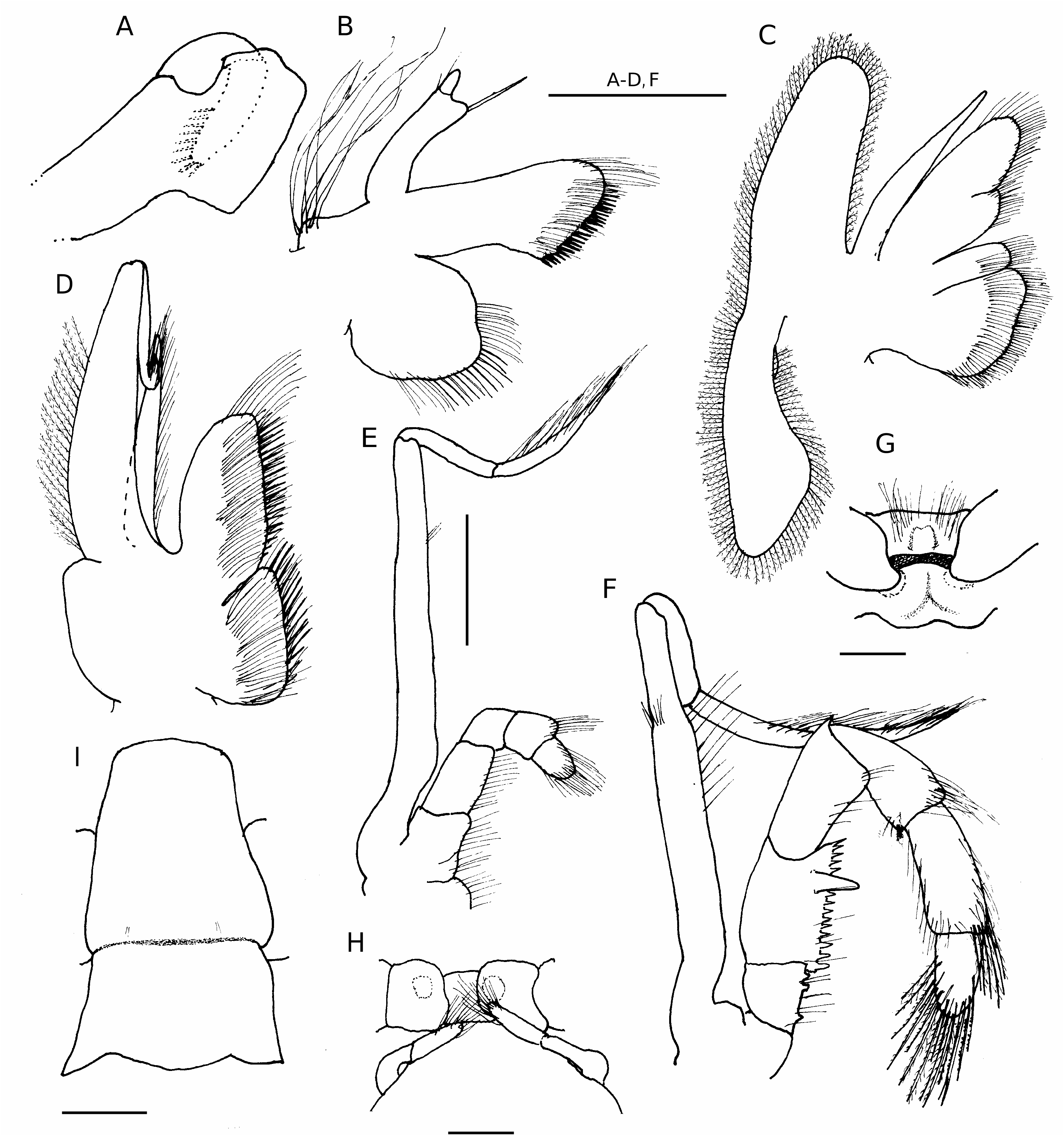
Thirteen pairs of quadriserial gills. Carapace lateral lobes very narrow, partially to completely calcified. Posteromedian and posterolateral carapace plates often only faintly delineated, membranous to weakly calcified. Rostrum triangular. Ocular acicles well developed, each with subterminal spine or spinule. Mandible ( Fig. 1A View FIG ) with incisor process margins calcified, lacking denticles. Maxillule ( Fig. 1B View FIG ) with external lobe of endopod moderately well developed, articulated, but not recurved, internal lobe with one bristle. Maxilla ( Fig. 1C View FIG ) with posterior lobe of scaphognathite elongate, slender. First maxilliped ( Fig. 1D View FIG ) with slender exopod lacking setae on distal 0.3 of outer margin. Second maxilliped ( Fig. 1E View FIG ) with elongate exopod. Third maxilliped ( Fig. 1F View FIG ) lacking fusion of basis and ischium; basis with two or three teeth; ischium with one accessory tooth on well developed crista dentata; merus with dorsodistal spine. Chelipeds grossly unequal, right largest. Sternite of third pereopods ( Fig. 1G View FIG ) with anterior and posterior portions separated by uncalcified hinge. Fourth pereopods semichelate; propodal rasp with two or three irregular rows of corneous scales; no preungual process. Male with paired second pleopods modified as gonopods ( Fig. 1H View FIG ); unpaired, unequally biramous left pleopods 3-5. Female with paired gonopores; no paired first pleopods; unpaired left pleopods 2-5. Abdomen straight, tergite of sixth pleomere ( Fig. 1I View FIG ) thickened, at least weakly calcified, and unequally subdivided by transverse suture; uropods symmetrical. Telson with transverse indentations delineating anterior and posterior portions; posterior lobes separated by distinct median cleft; concave terminal margins each with one to several small to moderately large spines.
AFFINITIES
Tomopaguroides View in CoL is, at least for the present, retained in the Pylopaguropsis View in CoL group of genera as proposed by de Saint Laurent-Dechancé (1966), sharing with the other nine currently assigned genera of the group the presence of 13 pairs of gills. However, Tomopaguroides View in CoL also shares with the unique genus, Pylojacquesia McLaughlin & Lemaitre, 2001 View in CoL of the family Pylojacquesidae McLaughlin & Lemaitre, 2001 View in CoL , a rather distinctive character mentioned only for one other member of the Pylopaguropsis View in CoL group, Chanopagurus Lemaitre, 2003 View in CoL . This is the broad separation of the anterior and posterior portions of the sternite of the third pereopods (thoracic sternite XII of McLaughlin & Lemaitre 2001). Pilgrim (1973) described this sternite as being divided by a membranous hinge, but this hinge is usually hardly observable. Despite familial differences, the overall similarities between Pylojacquesia View in CoL and certain genera of the Pylopaguropsis View in CoL group, as discussed by McLaughlin & Lemaitre (2001), lends support to the proposition by de Saint Laurent-Dechancé (1966) that this group is primitive within the family Paguridae View in CoL .
REMARKS
As noted by Provenzano (1968), among genera of the Pylopaguropsis group, Tomopaguroides appears most closely allied to Lithopagurus . Only species of these two genera are characterized as having paired second male pleopods modified as gonopods. This character, which sets the two genera apart not only from other genera of the Pylopaguropsis group but from all other genera of the Paguridae , appears to represent a progressive evolutionary loss that has occurred independently in the Diogenidae , Parapaguridae , Pylojacquesidae , and Paguridae , as well as in the galatheid family Porcellanidae . Among paguroids, male paired and modified first and second pleopods are characteristic of all genera of the Pylochelidae , but appear to be retained in the Diogenidae only the genera Paguropsis Henderson, 1888 , Paguristes Dana, 1851 , and Pseudopaguristes McLaughlin, 2002 . A fourth genus, Strigopagurus Forest, 1995 , apparently has already lost the modified, paired first pleopods and is undergoing evolutionary reduction in the second pleopods. This circumstance of the loss among the five species assigned to Strigopagurus was discussed in detail by Forest (1995: 107). Paired and modified first and second pleopods also are characteristic of the majority of genera of the Parapaguridae , however, reduction and/or absence in one or both pairs has been documented by Lemaitre (1996) for Oncopagurus Lemaitre, 1996 and Paragiopagurus Lemaitre, 1996 . Loss of male paired and modified pleopods is almost complete among pagurid genera. Only in species of Xylopagurus A. Milne Edwards, 1880 , are both paired and modified first and second pleopods found. One or two genera reportedly have rare occurrences of male paired first pleopods, and as previously indicated, only in Lithopagurus and Tomopaguroides are paired, modified second pleopods observed. No male paired and modified pleopods have been record- ed for Pylojacquesidae or any Lithodidae .
Tomopaguroides valdiviae ( Balss, 1911) ( Figs 1 View FIG ; 2 View FIG )
Parapagurus valdiviae Balss, 1911: 2 , fig. 2.
Tomopaguroides valdiviae – Balss 1912: 104, figs 14, 15, pl. 9, fig. 1.
? Tomopaguroides valdiviae – Provenzano 1968: 641 (table), 642 (key), 643 (key) (see Remarks).
TYPE MATERIAL. — Valdivia , stn 264, 6°18’N, 49°32’E, 1079 m, 30.III.1899, 2 syntypes ( ZMB 16470).
TYPE LOCALITY. — Indian Ocean off coast of Somalia, 6°18’N, 49°32’E, 1079 m.
DISTRIBUTION. — Eastern Africa, New Caledonia, Philippine, Salomon and Fiji islands.
MATERIAL EXAMINED. — Indian Ocean. Off Somalia, Valdivia , stn 264, 6°18’N, 49°32’E, 1079 m, 30.III.1899, 1 4.4 mm, lectotype, herein designated ( ZMB 16470).
New Caledonia. BATHUS 3, stn 793, 23°47’S, 169°48’E, 731-751 m, 26.XI.1993, 1 4.7 mm (MNHN-Pg 7064).
Philippine Islands. MUSORSTOM 1, stn 44, 13°46.9’N, 120°29.5’E, 610- 592 m, 24.III.1976, 7 4.3-6.3 mm, 2 specimens damaged, not sexed or measured (MNHN-Pg 7065) GoogleMaps ; stn 47, 13°40.7’N, 120°30.0’E, 757- 685 m, 25.III.1976, 5 4.6- 5.7 mm, 1 4.6 mm (MNHN-Pg 7066); 1 5.6 mm, 1 5.4 mm ( USNM 1016703 View Materials ) .
MUSORSTOM 2, stn 50, 13°36.7’N, 120°33.7’E, 810-820 m, 27.XI.1980, 1 4.1 mm (MNHN-Pg 7067); stn 79, 13°44.6’N, 120°31.6’E, 682-770 m, 1.XII.1980, 1 ovig. 5.1 mm (MNHN-Pg 7068); stn 82, 13°46.1’N, 120°28.4’E, 550 m, 2.XII.1980, 1 5.9 mm (MNHN-Pg 7069).
MUSORSTOM 3, stn CP 106, 13°47.0’N, 120°30.3’E, 668- 640 m, 2. VI.1985, 1 3.9 mm (MNHN-Pg 7070).
Salomon Islands. SALOMON 1, stn CP 1858, 9°37.9’S, 150°40.7’E, 435-461 m, 7.X.2001, 2
3.3, 4.0 mm (MNHN-Pg 7071).
Fiji Islands. MUSORSTOM 10, stn CP 1343, 16°38.95’S, 177°40.84’E, 938-940 m, 10.VIII.1999, 1 ovig. 4.5 mm (MNHN-Pg 7072).
BORDAU 1, stn CP 1415, 16°31’S, 179°00’W, 670- 682 m, 27.II.1999, 1 4.2 mm (MNHN-Pg 7073); stn CP 1419, 17°05’S, 178°55’W, 654-656 m, 26.II.1999, 2 4.2, 5.1 mm, 1 4.6 mm (MNHN-Pg 7074); stn CP 1502, 18°21’S, 178°27’W, 640-660 m, 13.III.1999, 1 4.4 mm, 1 ovig. 5.3 mm (MNHN-Pg 7075).
BORDAU 2, stn DW 1508, 21°02’S, 175°19’W, 555-581 m, 31. V.2000, 1 3.1 mm (MNHN-Pg 7076).
HABITAT. — Appears to be exclusively shells of the scaphopod family Dentalidae . At least specimens of two genera, Fissidentalium Fisher, 1885 and Compressidentalium Habe, 1963 could be recognized from the Philippine collections.
REDESCRIPTION
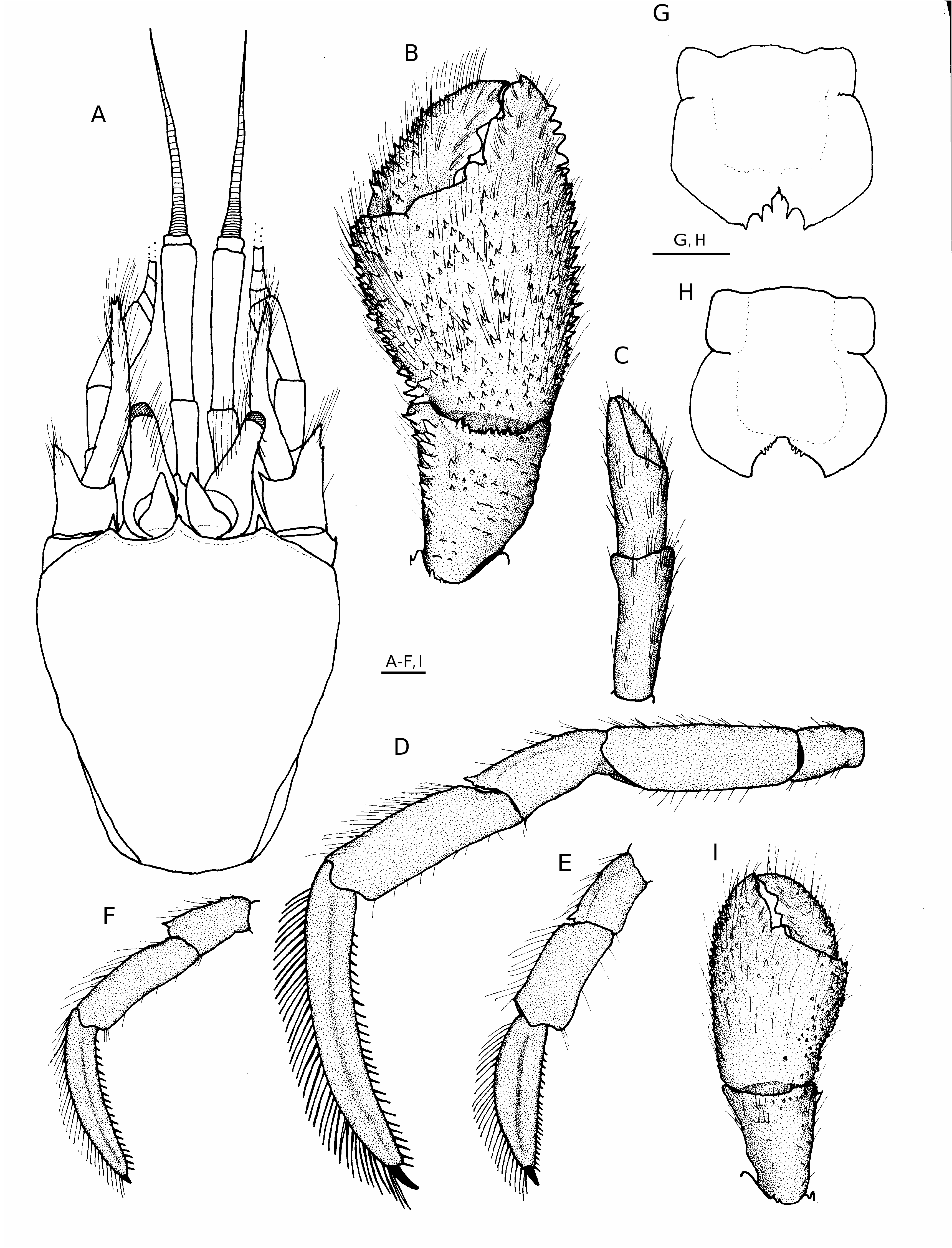
Shield ( Fig. 2A View FIG ) somewhat vaulted; considerably longer than broad; anterior margin between rostrum and lateral projections concave; anterolateral margins sloping; posterior margin roundly truncate; dorsal surface well calcified, slightly rugose and pitted but glabrous. Rostrum acutely triangular, produced beyond bases of ocular acicles but often not beyond level of lateral projections, terminating with spinule or spine. Lateral projections well developed, acute, subacute or blunt, each with prominent marginal or submarginal spine, occasionally with both marginal and submarginal pair of spines.
Ocular peduncles very short, 0.3-0.4 shield length, stout basally and tapering to reduced corneas, dorsomesial surfaces each often somewhat flattened or depressed in distal 0.4-0.5, dorsal or dorsomesial surfaces each with tufts or row of setae; corneas small, diameter 0.1-0.2 of peduncular length. Ocular acicles elongate, thick, triangular, dorsally flattened in distal 0.3-0.5, each with tiny terminal spinule; closely-set basally, divergent distally.
Antennular peduncles overreaching ocular peduncles by 0.1 to entire length of penultimate segment. Ultimate and penultimate segments each with few long setae dorsally. Basal segment with slender, acute spine on dorsolateral margin.
Antennal peduncles overreaching distal corneal margins by 0.1-0.5 lengths of fourth peduncular segments. Fifth and fourth segments each with numerous scattered setae. Third segment with sparse tuft of long setae at ventrodistal margin, occasionally concealing tiny spine or spinule. Second segment with dorsolateral distal angle produced, terminating in bifid or simple spine and frequently with accessory subdistal spine, dorsal surface commonly with transverse, often spinulose ridge and long setae, spine, when present at lateral margin, usually most prominent; dorsomesial distal angle with moderately small to large spine. First segment usually with small spine at laterodistal margin; ventral margin with two to five small to moderately large spines distolaterally. Antennal acicle overreaching distal margins of corneas by 0.3-0.7 own length and often reaching to or slightly overreaching distal margin of ultimate peduncular segment, slightly arcuate, with row of long, moderately dense setae on mesial margin and similar row on distal 0.5 of lateral margin; terminating in simple spine. Antennal flagella moderately long, but usually not overreaching outstretched right cheliped; each article with several irregularly set, moderate to long (four to six article-length) setae.
Right cheliped ( Fig. 2B View FIG ) much larger than left, operculate or nearly so. Dactyl broad, slightly shorter to slightly longer than dorsomesial margin of palm; articulation with chela usually somewhat oblique; cutting edge with three large, prominent to well-worn calcareous teeth and with very short row of tiny corneous teeth adjacent to terminal corneous claw; dorsal surface generally flat, with scattered tufts of moderately long setae and few small spines proximally, dorsomesial margin with abundance of long setae not concealing two to four irregular rows of moderate to small spines, decreasing in size distally; mesial face with low, sometimes spinulose protuberances and tufts of moderately short setae frequently forming irregular and incomplete vertical rows; ventromesial margin not delineated, ventral surface with few sparse tufts of very short setae. Palm 1.0-1.4 longer than carpus; dorsomesial margin delimited by irregular double to triple row of moderate to small, blunt to acute spines, dorsal surface with covering of long setae not obscuring armature, usually one row of widelyspaced smaller spines adjacent to dorsomesial margin, remainder of dorsal surface with irregular rows of small spines, most numerous laterally but often forming weak, inverted V in midline, dorsolateral margin with single to double row of small spines, usually larger on fixed finger and extending nearly to tip; flattened dorsal surface of fixed finger with numerous long setae and often with few small spines, tubercles or low protuberances; mesial and lateral faces each often with numerous small spines and long setae, at least dorsally; ventromesial and ventrolateral margins not delimited, rounded ventral surface with few very low protuberances and scattered short setae; cutting edge of fixed finger with one blunt tooth proximally, one broad tooth medianly and very weakly cusped, calcareous margin distally. Carpus approximately equal to length of merus, carpal/meral articulation rotated clockwise 15- 25° from perpendicular; dorsodistal margin unarmed or with one to several small spines or spinules, sometimes extending onto laterodistal margin, accompanied by several long setae, dorsomesial margin with single to double row of moderate to small, acute or subacute spines and few long setae, dorsal surface mesiad of midline with distal 0.3 forming slight to distinct concavity or basin, and usually armed with several scattered small spines or tubercles, remainder of dorsal surface weakly convex and with scattered small spines, tubercles or protuberances; dorsolateral margin not delimited, lateral, mesial and ventral surfaces each with few low protuberances and sparse short setae. Merus roundly subtriangular; dorsal margin unarmed; distal margin with one prominent spine mesially and often one to five smaller spines dorsally; ventrolateral margin with row of widely-spaced minute spinules or granules; ventromesial margin with row of very small or small spines, largest in distal 0.3; ventral surface often with scattered granules, tubercles or spinules. Ischium unarmed. Coxa with small spine at ventromesial distal angle and frequently one or two spines on ventrolateral distal margin.
Left cheliped ( Fig. 2C View FIG ) short, slender; rotation of propodal-carpal articulation 5-30° counterclockwise from perpendicular. Combined length of dactyl and palm approximately equal to individual lengths of carpus and merus; all segments unarmed but with sparse rows of tufts of long setae, most numerous on dorsal surfaces of chela and carpus.
Ambulatory legs ( Fig. 2 View FIG D-F) generally similar. Dactyls 1.2-1.8 length of propodi, maximum dorsal-ventral height 0.1-0.2 of total length; dorsal surfaces each with row of tufts of moderately dense, long setae; lateral and mesial faces each with faint to prominent longitudinal sulcus, flanked on mesial face dorsally and ventrally by row of sparse tufts of setae, lateral face with few tufts of short setae; ventral margins each with row of 15-26 corneous spines. Propodi 1.2-1.4 longer than carpi, each unarmed but with tufts of long setae dorsally and ventrally, fewer in number ventrally. Carpi 0.5-0.8 length of meri; dorsodistal angles each with small spine, dorsal surfaces varying from each with only sparse tufts of moderately long setae to two moderate to small spines in proximal half. Meri with scattered setae on dorsal and ventral margins, dorsodistal margins unarmed or each with small spine. Ischia unarmed but with sparse setae on ventromesial margins. Coxae each usually with one to five small spines on ventromesial distal margin. Fourth pereopods with propodal rasp consisting of two or three rows of corneous scales; dactyl with small terminal claw, no preungual process. Anterior lobe of sternite of third pereopods subtriangular to subquadrate, unarmed or with one to several small spines marginally, usually concealed by long setae.
Abdomen elongate, straight; tergite of sixth pleomere ( Fig. 1I View FIG ) divided into unequal halves by deep transverse suture; proximal half subtriangular, moderately well calcified; distal half subquadrate or subrectangular, well calcified, posterolateral angles each somewhat to prominently produced, terminally subacute, with or without spine. Males with two-segmented paired second pleopods modified as gonopods ( Fig. 1H View FIG ); distal segments each with terminal and/or subterminal dense tufts of long setae; pleopods 3-5 with exopods moderately well developed, endopods rudimentary, both with sparse marginal setae. Females without paired, modified first pleopods; pleopods 2-4 with both rami well developed, exopod slightly longer; pleopod 5 as in males.
Telson ( Fig. 2G, H View FIG ) with broadly rounded, symmetrical posterior lobes separated by narrow, moderately shallow median cleft; terminal margins concave, each with one to three moderately large, often corneous-tipped spines and usually three to six smaller spines adjacent to cleft, occasionally with only two or three small spines or tubercles adjacent to cleft.
COLOUR ( IN LIFE)
Not known. No colour remaining in preserved specimens.
COMPARISON OF LECTOTYPE WITH BALSS’ ILLUSTRATIONS
Differences in the relative sizes of the corneas and ocular peduncles, armature of the right chela and carpus, and the positions of the left cheliped and ambulatory legs suggest that the syntype illustrated by Balss (1911: fig. 2; copied in 1912 as fig. 14) is not the same specimen as the syntype illustrated by the artist W. Engles ( Balss 1912: pl. 9, fig. 1), although, these differences might simply reflect “artistic license” in the latter figure. The shapes of the ocular peduncles and corneas of the examined syntype, herein designated as the lectotype, would suggest that it is this specimen that was depicted in Balss’ line drawing (1911: fig. 2; 1912: fig. 14); however, his measurement of 9 mm for carapace length corresponds well with the specimen illustrated in plate 9, figure 1, and is considerably greater than the actual 7.3 mm measured carapace length of the lectotype.
The supposition that Balss’ (1911: fig. 2; 1912: fig. 14) line drawing is of one syntype, while the artist’s rendition ( Balss 1912: pl. 9, fig. 1) is of the other is given additional credence by the fact that only Balss’ (1911: fig. 2) figure of the anterior portion of the cephalothorax and appendages appeared in his initial description of Parapagurus valdiviae . It was after that preliminary publication that Balss apparently realized that parapagurids did not have 13 pairs of gills and that the gill number and other characters seemed to relate this species to Tomopaguropsis Alcock, 1905 . Although his specific description remained identical with that of Parapagurus valdiviae , in Balss’ (1912: 104) generic diagnosis he expressed his uncertainty as to whether the paired gonopods were on the second or first pleonal somite, but suspected the second. For Tomopaguroides valdividae, Balss (1912 : fig. 15) included the line drawing of the ventral view of the thorax and anterior portion of the abdomen together with his earlier figure of the cephalothorax and appendages. In the later publication Balss also added full plates depicting several species drawn by artists. Neither the ventral view of the thorax (fig. 15) nor the illustrated entire animal (pl. 9, fig. 1) of T. valdiviae correspond well with the lectotype.
There are several morphological differences between the lectotype and the illustrated specimen(s) that may reflect some factual variations, but also clearly indicate certain incorrect observations and inaccuracies in the original figures. The rostra illustrated in both the line drawing and the plate figure, for example, are much shorter than the tips of the lateral projections and lack a terminal spine. And in his generic diagnosis Balss (1912: 104) specifically noted a similarity between the rostrum of Tomopaguroides valdiviae and the short rostra of the two species of Tomopaguropsis known at the time. However, the rostrum of the lectotype is more acutely triangular than illustrated and has a prominent terminal spine that extends the rostral length to only slightly less than those of the lateral projections. The antennal acicles were not described by Balss, but those of the lectotype are longer than those shown in either of Balss’ figures. The illustrated antennal flagellum ( Balss 1911: fig. 2; 1912: fig. 14) shows each article with a pair of setae of equal length; whereas, the specimen illustrated in Balss’ (1912) plate 9, figure 1 shows no setation at all. The articles of the antennal flagella of the lectotype each has several irregularly set, moderate to long setae. The armature of the right cheliped as portrayed by Balss (1911: fig. 2; 1912: fig. 14, pl. 9, fig. 1) does not agree with that of the lectotype. Although a spinose dorsomesial margin of the chela is depicted relatively accurately, the dorsal surface of the chela of the lectotype has a covering of small spines, but is shown with only setae in the illustrations. In the lectotype, the spines on the dorsolateral margin extend nearly to the proximal margin of the chela as illustrated in Balss’ (1912) plate 9, figure 1, but not figure 14. The spines on the dorsomesial margin of the carpus of the lectotype extend the entire length of that margin, rather than only half the distance as in both figures 14 and plate 9, figure 1. On neither pair of ambulatory legs is a dorsodistal carpal spine indicated on either of Balss’s figures; however, the carpi of the lectotype each has a small but distinct dorsodistal spine.
COMPARISON OF THE LECTOTYPE WITH PACIFIC SPECIMENS
Although there is variation in rostral development, the lectotype agrees well with the Pacific specimens, if not with Balss’ (1911: fig. 2; 1912: fig. 14, pl. 9, fig. 1). Similarly, the ratios of ocular peduncular length to shield length and corneal diameter to peduncular length are within the ranges of the Pacific populations. The antennular and antennal peduncles of the lectotype both overreach the distal corneal margins by approximately 0.5 length of the penultimate segments, lengths also with in the ranges exhibited by the Pacific specimens. The produced dorsodistal angle of second segment of the antennal peduncle lacks an accessory spinule in the lectotype and the ridge on the dorsal surface of the segment is not spinulose; however, the presence of an accessory spine or spinules on the ridge was found to be variable in the Pacific populations. The length of the antennal acicles of the lectotype are well within the range seen in the Pacific specimens. As noted above, the articles of the antennal flagella of the lectotype each have several irregularly set, moderate to long setae like those of its Pacific counterparts. The spination on the dorsal surface of the right chela common to the lectotype and the Pacific specimens was apparently unnoticed by Balss.
Comparisons of the ratios of dactylar length to height and dactylar to propodal lengths of the second left pereopod of the lectotype have shown that both ratios fall well within the ranges found in the Pacific populations, i.e. 0.1 and 1.6 respectively. Similarly, the longitudinal sulci on the lateral faces of the dactyls, although weak, are within the range of variation seen among the Pacific representatives. However, in the number of spines on the ventral margins of the dactyls, only 15 are present in the lectotype, whereas the range is 16-26 in the Pacific specimens. The carpi of the ambulatory legs of the lectotype each have the typical small dorsodistal spine as previously noted, but dorsal carpal surfaces show only low protuberances and sparse setae. Carpal armature in Pacific specimens varied from only sparse setae to two moderate to small spines in the proximal half of the segment. In the lectotype, the coxae of these appendages are armed with two or three small spinules on the second pereopods, but zero to one on the third. The fourth pereopods of the lectotype have two rows of corneous scales, and the subtriangular anterior lobe of the sternite of the third pereopods has two small, subacute terminal spinules.
Balss (1912: fig. 15) illustrated the paired second male pleopods as being crossed. These appendages in the lectotype ( Fig. 1H View FIG ) are not crossed, nor were they in any of the males of the Pacific populations. The telson of the lectotype ( Fig. 2H View FIG ) lacks the small spinules seen on telsons of some, but not all Pacific specimens. Reexamination of the lectotype has confirmed the identifications of the Pacific populations as belonging to Tomopaguroides valdiviae .
SEX RATIO AND REPRODUCTION
A single male was collected in New Caledonian waters. Males were much more common in the samples from the Philippines Islands with a ratio of four males to every one female. Of those four females collected during cruises in Philippine waters in 1976, 1980 and 1985, only one was an ovigerous female taken in March. In contrast, the sex ratio in the Fiji area was evenly balanced, with four males and four females recovered. Of the females, two of the four were ovigerous, with one collected in February and the second in August. The eggs carried by females from both areas were large and relatively few in number, although because of the ease with which eggs could be dislodged from the pleopods, an actual egg count was not reliable. Egg diameters measured between 1.1 and 1.4 mm at early stages of development (non-eyed).
VARIATIONS
As may be ascertained from the character variability incorporated into the redescription, Tomopaguroides valdiviae is another hermit crab taxon with considerable morphological plasticity. The differences between specimens collected in the Philippines and those from Fiji initially suggested two distinct taxa. Fortunately , MUSORSTOM collections from a broad area of the western Pacific were available for simultaneous examination and these contained a sufficient number of specimens to make an accurate assessment of intraspecific variation possible. Most striking were the variations seen in the length/depth ratios of the ambulatory dactyls and the armature of the carpi and meri of those appendages ( Fig. 2 View FIG D-F). The dactyls of the second and third pereopods of the lectotype and Philippine specimens varied from 1.5 to 1.8 times the length of the propodi with maximum dorsalventral height of approximately 0.1 of the length, whereas, some specimens from Fiji had much shorter dactyls (1.2-1.3 length of propodi) with the maximum dorsal-ventral height 0.2 of the length. The carpi of the lectotype and most of the Philippine specimens carried only a dorsodistal spine, although setae-bearing protuberances were present on the lectotype and occasionally could be discerned in the posterior halves of the dorsal margins of the Philippine specimens. In contrast, in the majority of specimens from Fiji, the dorsal margins of the carpi had at least one and more often two well developed spines on the posterior half. The ambulatory meri of all of the Philippine specimens had unarmed dorsodistal margins as did the lectotype, while the dorsodistal margins of these segments in some, but not all Fiji specimens were each armed with a small spine. Additional significant variations were observed in the lengths of the antennular peduncles (shorter in the Fiji specimens) and lengths of the ocular peduncles (longer in the Fiji specimens). That these differences reflected within population variations rather than specific differences were confirmed, in part, by the two specimens from the Salomon Islands and the single specimen from New Caledonia. Although the larger of the two Salomon Islands males had abnormal cheliped development, both specimens lacked posterior spines on the dorsal margins of at least one or two of the four pereopodal carpi ; the meri all were unarmed. The New Caledonia male lacked posterior carpal and dorsodistal spines on the segments of all pereopods. The lengths of the dactyls and the maximum dorsal-ventral heights were intermediate between those of the Philippine and Fiji specimens. The lengths of the ocular peduncles of the New Caledonia and Salomon Islands males approximated those of the Philippine specimens, while the lengths of the antennular peduncles of the Salomon Islands males were comparable to those of the Fiji material. Further evidence was provided by the examination of one Philippine specimen in too poor condition to be included in the species’ evaluation. That specimen had a small dorsoproximal spine on each pereopodal carpus. Variations in the prominence of the longitudinal sulci on the dactyls of the ambulatory legs were also noted ; however, these variations did not seem to be zoogeographically influenced. From the information presently available, these variations do not appear to be correlated to either size or sex.
The abnormal cheliped development of male specimen from the Salomon Islands previously mentioned involved a presumed regeneration anomaly. That individual had no left cheliped. In its position was a fully developed, albeit reversed, right cheliped ( Fig. 2I View FIG ). On the animal’s right side was a smaller right cheliped, correct in form, but with appreciably weaker spination on the chela and carpus than was present on the cheliped on the left side. In all other respects the specimen appeared normal.
AFFINITIES
There is a very cogent, albeit superficial, resemblance between females of Bathypaguropsis microps ( Balss, 1911) and females of Tomopaguroides valdiviae . In addition to sharing the general characters of the Pylopaguropsis group of genera in terms of gills, both have very short ocular peduncles with reduced corneas, thick, dorsally somewhat flattened, triangular ocular acicles, grossly unequal chelipeds, relatively unarmed ambulatory legs with somewhat laterally compressed dactyls, and broadly rounded posterior terminal telsonal lobes. And both species occur sympatrically. Balss (1911) described T. valdiviae (as Parapagurus ) and B. microps (as Eupagurus ) from the same Valdivia station off the coast of Somalia. In the present study, a single female of B. microps was collected at MUSORS- TOM Philippine station 44, with nine specimens of T. valdiviae . Had McLaughlin (2003c) not reexamined the male holotype of B. microps and determined that Balss (1911, 1912) had erred in his report of the gill number and lamellar type, it is very possible that the female specimen from MUSORSTOM Philippine station 44 might incorrectly have been described as a new species of Tomopaguroides .
REMARKS
As previously pointed out, character states attributed to Tomopaguroides valdiviae by Provenzano (1968) do not agree, in some particulars, with those of the specimens included in this investigation. Most important is the reported presence of rudimentary paired first pleopods in females, and referred to by Provenzano as gonopods. None of the eight females examined in the present study exhibited any development of first pleopods. Therefore, if the female specimen(s) that de Saint Laurent described through Provenzano (1968) had rudimentary paired first pleopods, it can only be assumed that she was incorrect in her assessment of the species as T. valdiviae . Paired and modified female first pleopods do occur in five genera of the Pylopaguropsis group, Bythiopagurus McLaughlin, 2003 , Chanopagurus , Munidopagurus A. Milne-Edwards & Bouvier, 1893 , Pylopaguropsis Alcock, 1905 , and Xylopagurus A. Milne Edwards, 1880 , but only in Chanopagurus atopos Lemaitre, 2003 , are the corneas reduced as they are in Tomopaguroides valdiviae . Since females of C. atopos have a single left gonopore while those of T. valdiviae have paired gonopores, it is unlikely that de Saint Laurent would have confused those two taxa.
Provenzano’s (1968: 641) table 1 indicated that dorsal spines were absent from the carpi of both pairs of ambulatory legs of T. valdiviae , but whether his information was gleaned from de Saint Laurent’s unpublished information or Balss’ (1911: fig. 2; 1912: fig. 14, pl. 9, fig. 1) was not specified. However, the lectotype and all of the 31 Pacific specimens in the current collection had at least a dorsodistal spine on each carpus. One specimen from the Philippines and all specimens from Fiji and the Salomon Islands also had at least one, and often two, dorsoproximal spines on one or more carpi. Differences in other attributes, such as rostral length and ocular acicle development, fall well within the ranges of variation seen for T. valdiviae . Provenzano cited only one measurement of specimen size and that was the 9 mm carapace length given by Balss (1911, 1912). As previously indicated, the lectotype has a shield length of 4.4 mm and a carapace length of 7.3 mm. Specimens in the Pacific series ranged in shield length from 3.1 to 6.3 mm. Shield length in T. valdiviae varies from 0.6 to 0.7 of carapace length, thus only the largest of these specimens approached the size specified by Balss and reflected in his illustration (1912: pl. 9, fig. 1) of the entire animal.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Tomopaguroides Balss, 1912
| Mclaughlin, Patsy A. 2004 |
Tomopaguroides valdiviae
| PROVENZANO A. J. JR. 1968: 641 |
Tomopaguroides
| MCLAUGHLIN P. A. 2003: 128 |
| GORDAN J. 1956: 342 |
| BALSS H. 1912: 104 |
Tomopaguroides valdiviae
| BALSS H. 1912: 104 |
Parapagurus valdiviae
| BALSS H. 1911: 2 |