Cernosvitoviella farkasi, Dózsa-Farkas, Klára, Csitári, Bianka & Felföldi, Tamás, 2017
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4254.3.2 |
|
publication LSID |
lsid:zoobank.org:pub:6F7301AD-20E4-48B5-A143-499FCAE083B3 |
|
DOI |
https://doi.org/10.5281/zenodo.6029228 |
|
persistent identifier |
https://treatment.plazi.org/id/611E1238-FFA3-FFFC-FF56-F4879826F87B |
|
treatment provided by |
Plazi |
|
scientific name |
Cernosvitoviella farkasi |
| status |
sp. nov. |
Cernosvitoviella farkasi View in CoL sp. n.
( Figures 1 View FIGURE 1 A, 2–4)
Holotype. C4 slide No.1064, adult, stained whole mounted specimen.
Type locality. Kȏszeg Mts. , near to the Sphagnum mire, 47o24.180N 16o33.531E, 349 m asl, in a young Scots pine forest with Molinia , mud, leg. K. Dózsa-Farkas, J. Farkas, Z. Tóth, 21.05.2014. GoogleMaps
Paratypes (in total 59 specimens). P.89.1–89.13 slides No 1037, 1039–1042, 1044–1045, 1062–1063, 1065, 2150–2151, 2220, 19 stained specimens from type locality, 21.05.2014. P.89.14, 19 specimens from type locality, in 70 % ethanol, 21.05.2014. P.89.15, 11 specimens from type locality, 13.10.2014. P.89.16, 10 specimens from type locality, in 70 % ethanol, 24.10.2016.
Further material examined. 22 living specimens, not fixed, from the type locality.
Etymology. Named in honour of our colleague, Dr. János Farkas, who assisted many times in recent sampling campaigns.
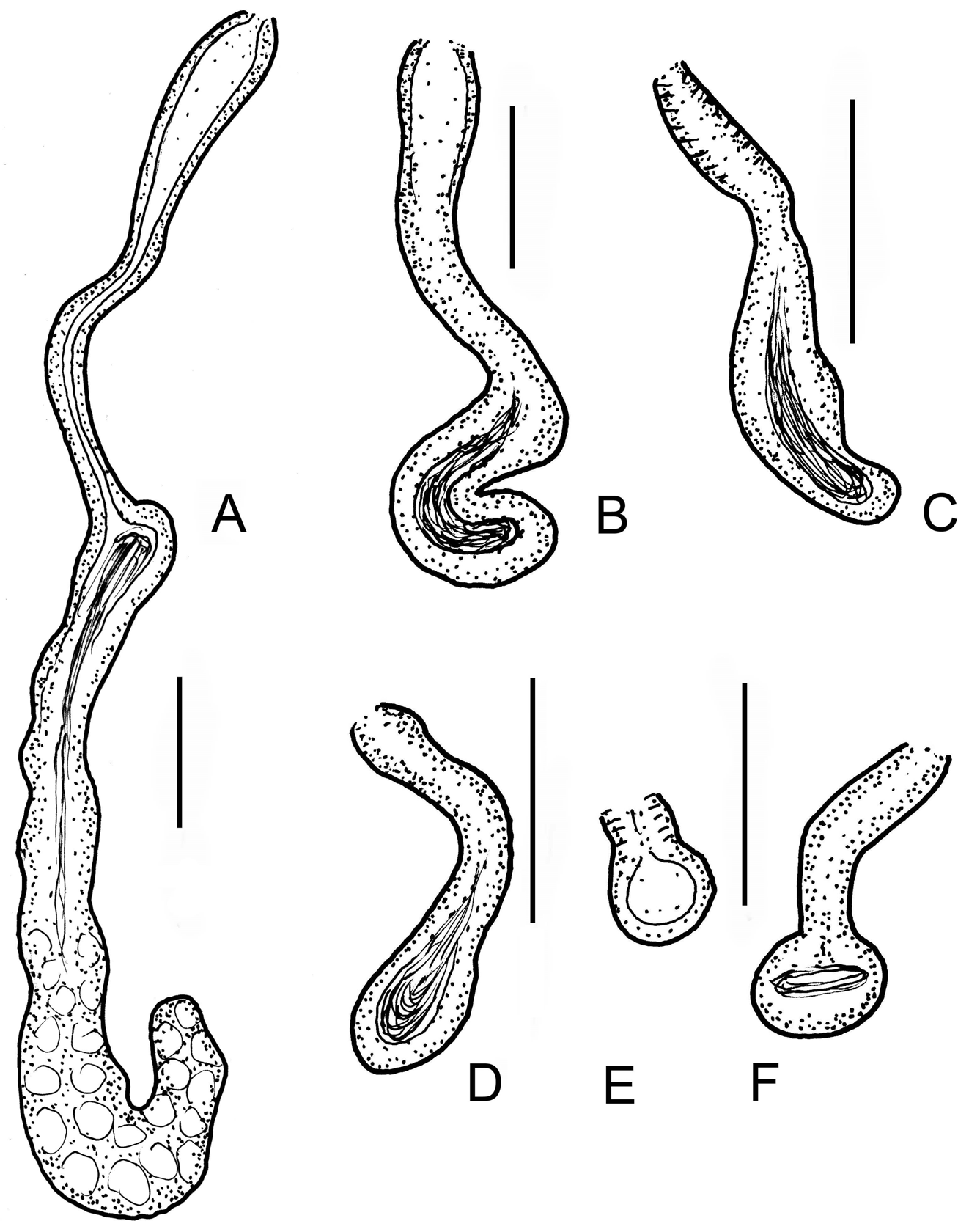
Diagnosis. The new species can be recognized by the following combination of characters: (1) small size (body length 3–5 mm, in vivo), segments 22–26; (2) maximum 6–8 sigmoid and nodulate chaetae per bundle; (3) clitellum developed only laterally; (4) two or three unpaired nephridia preclitellarly; (5) coelomocytes spindleshaped, with refractile granules, black under transmittent light; (6) 2 + 2 pharyngeal glands; (7) sperm funnel cylindrical, large, approximately 2/3 as long as body diameter, collar conspicuous, slightly narrower than the funnel body; (8) sperm ducts considerably widened in the middle; (9) male copulatory organs large, pores surrounded by gland cells; (10) spermathecae free, reaching VII–IX segments, consisting of ectal ducts, hemispherical parts with sperm and long wide ampullae; the distal part of ectal ducts with conspicuous widenings.
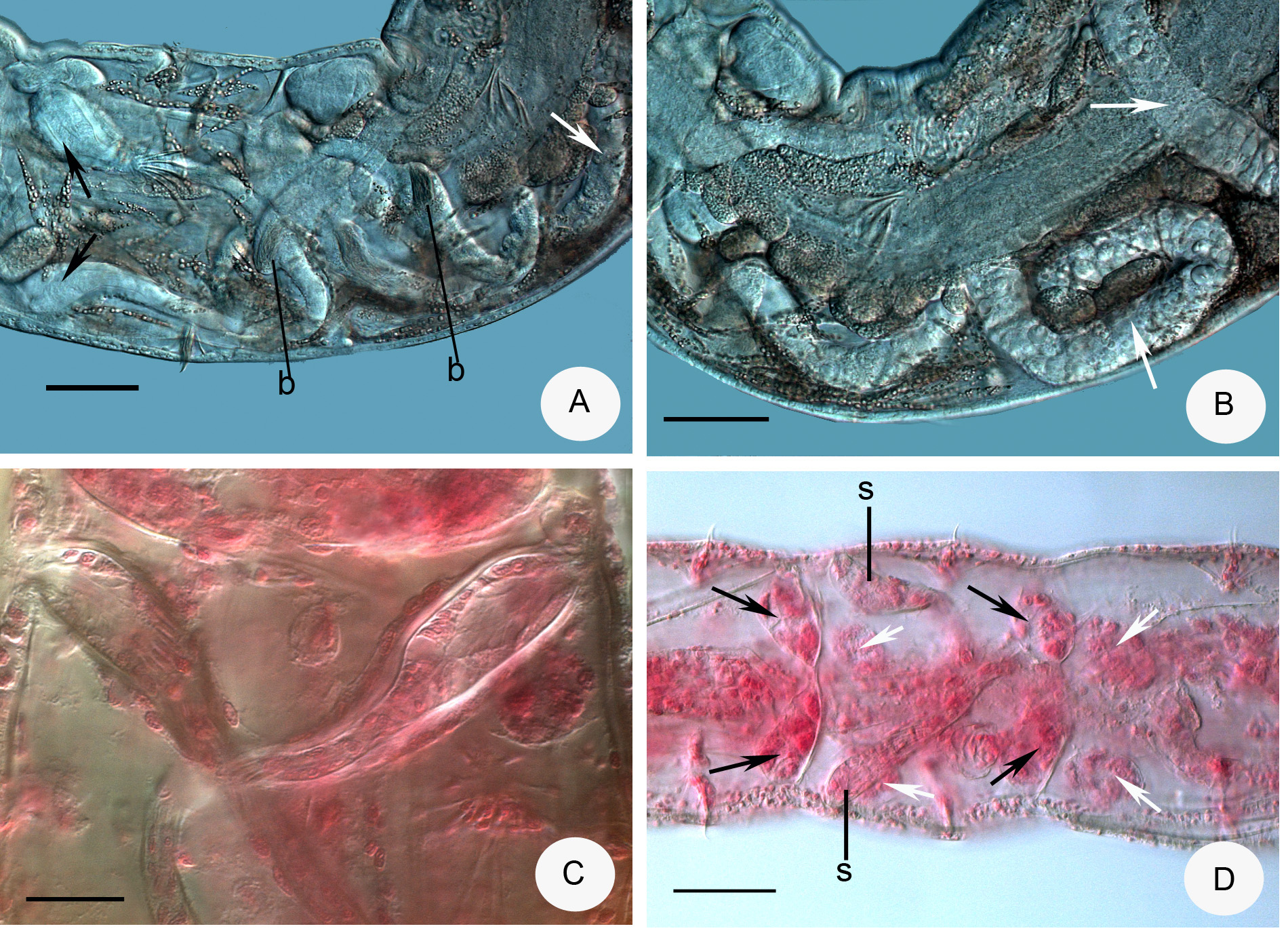
Description. Holotype 3.0 mm long, 160 µm wide at VIII and 205 µm at clitellum (fixed), 25 segments. Length of paratypes 3–5 mm, width 140–230 µm at VIII and 200–310 µm at clitellum in vivo, length of fixed specimens 1.8–3.8 mm, width 130–160 µm at VIII and 170–220 µm at clitellum, segments 22–26. Chaetae slender, sigmoid, with nodulus; number of chaetae per bundle variable, up to 6–8 in ventral preclitellar bundles, length 28– 35 µm. Chaetae in XII absent. Head pore at 0. Clitellum only laterally in XII–1 /2XIII, gland cells in dense rows or irregularly distributed (granulocytes about 10–13 µm long and 7–8 µm wide (fixed), the hyalocytes slightly smaller and fewer). Thickness of body wall about 12 µm in vivo, (9 µm when fixed), cuticle very thin. Brain deeply cleft posteriorly, about 100 µm long and two times longer than wide when fixed ( Fig. 2 View FIGURE 2 A). In prostomium, about 12 inner papillae ( Figs. 2 View FIGURE 2 A,B) similar to those found in Xetadrilus ( Schmelz et al. 2011) . This trait of Cernosvitoviella species is mentioned for the first time here. Oesophageal appendages and intestinal diverticula absent. Two pairs of primary pharyngeal glands in 4/5 and 5/6, dorsally without or with narrow connection, two secondary glands free in V and VI. ( Fig. 4 View FIGURE 4 D). Chloragocytes from V and forming a dense layer from VI, individual cells relatively large (22–55 µm long) and containing refractile oil droplets in vivo ( Fig. 2 View FIGURE 2 G). Midgut pars tumida in XV–XIX, occupying 2–3 segments ( Fig. 2 View FIGURE 2 E). Dorsal vessel from XII or in front of XIII, blood slightly pink, the anterior bifurcation in peristomium. 2–3 mostly unpaired preclitellar nephridia from 6/7 or 7/8, anteseptale small with funnel only, postseptale with conspicuous canals, efferent duct terminal ( Fig. 2 View FIGURE 2 F). Coelomocytes spindle-shaped with dark refractile granules, 36–60 µm long, 7–8 µm wide in the middle in vivo ( Figs. 2 View FIGURE 2 C,D). In fixed specimens they are only 20–22 µm long, the granules are not visible but the nucleus is large. Seminal vesicle large in X–XI ( Fig. 3 View FIGURE 3 A). Sperm funnels cylindrical, large ( Figs. 2 View FIGURE 2 I, 3A,C), about 90–180 µm long in vivo (80–155 µm, fixed) and 2–3 times longer than wide, about 3/4 of body diameter; collar distinct, tall and slightly narrower than the funnel body. Spermatozoa about 70 µm long, heads 25 µm, in vivo (40–60 µm and 10–20 µm, fixed, respectively). Sperm ducts considerably widened in the middle ( Figs. 2 View FIGURE 2 I, 3B,C,D). This thick-walled dilation about 120–180 µm long, 30–50 µm wide and the canal is 17–26 µm wide in vivo (100–180 µm, 23–34 µm and 14–17 µm, fixed, respectively). Tracts of the sperm duct before and after the dilation of about the same length as the dilated part; just after the sperm funnel duct is slightly thinner, diameter about 8–13 µm until the dilation, while the part after the dilation is 13–19 µm wide, finally slightly dilated again in the male copulatory organ, up to 15–20 µm in vivo. Male copulatory organs large, the male pore surrounded by glands forming a round and compact mass ( Figs. 3 View FIGURE 3 B,C,D,E), diameter 60–90 µm in vivo (60–70 µm, fixed). Subneural glands absent. Spermathecae free ( Figs. 1 View FIGURE 1 A, 2H, 4A.B,D), ectal glands absent. Spermathecae consist of ectal ducts (about 170 µm long and 10 µm wide in vivo, and fixed alike) widened ectally to up to 25 µm in vivo (20 µm, fixed) ( Figs. 3 View FIGURE 3 F,G, 4C). The ectal ducts in VI widens into almost hemispherical parts with sperm in them (25–32 µm wide in vivo and fixed alike) ( Figs. 3 View FIGURE 3 G. 4A). These bulbiform parts continue in long ducts which expand into wide sack-like ampullae (30–50 µm wide, in vivo and fixed alike). Ampullae reaching VII–IX segments when fully developed ( Figs. 2 View FIGURE 2 H, 4A,B). In the subadult specimens the hemispherical parts absent and the ampullae smaller, expanding only to VI or VII. One mature egg at a time. On the body wall surface, often epibiotic ciliates attached, similar to other Cernosvitoviella species ( Figs. 2 View FIGURE 2 I, 3H).
Distribution and habitat. Known only from the type locality.
Differential diagnosis. The new species is clearly distinguished from the rest of hitherto described Cernosvitoviella species by the prominent dilatation in the middle part of the vas deferens. C. farkasi sp. n. is most similar to C. aggtelekiensis in size, the type of coelomocytes, and the remarkable dilatations of the vasa deferentia ( Figs. 3 View FIGURE 3 B–D vs. Figs. 5 View FIGURE 5 I,J). However, in the new species coelomocytes are narrower ( Figs. 2 View FIGURE 2 C,D vs. Figs. 5 View FIGURE 5 E,F), the dilatations of the vasa deferentia are located more proximally, and they are more conspicuous and refracting. The spermathecal ectal duct of C. aggtelekiensis has also a widening distally, but the ampullae reach only into V or VI ( Figs. 1 View FIGURE 1 B, 6D–F). In five Cernosvitoviella species ( C. sphaerotheca Healy, 1975 , C. briganta Springett 1969 , C. palustris Healy, 1979 , C. estaragniensis Giani, 1979 and C. ampullax Klungland & Abrahamsen, 1981 ), the spermathecae also extend into VIII or IX, but in these species the dilatations of the vasa deferentia are absent or smaller, or they occur distally if present.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |