Marphysa iloiloensis, Glasby & Mandario & Burghardt & Kupriyanova & Gunton & Hutchings, 2019
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4674.2.7 |
|
publication LSID |
lsid:zoobank.org:pub:0DEB565C-E5C5-4AA9-A76F-DB017B4F64C6 |
|
persistent identifier |
https://treatment.plazi.org/id/03FC87B9-E068-0A72-FF26-EFBAD7AE1D01 |
|
treatment provided by |
Plazi |
|
scientific name |
Marphysa iloiloensis |
| status |
sp. nov. |
Marphysa iloiloensis View in CoL n. sp.
( Figs 1 View FIGURE 1 –9; Table 3 View TABLE 3 )
Material examined. HOLOTYPE, NTM W29624: Marine Annelids Hatchery, Southeast Asian Fisheries Development Center, Aquaculture Department ( SEAFDEC- AQD), (10°40’25.284”N, 122°22’35.328”E), Brgy. Buyu-an, Municipality of Tigbauan , Iloilo Province, Western Visayas, Philippines. coll. Mary Anne E. Mandario, June 15, 2017. GoogleMaps
PARATYPES: NTM collection : NTM W29619 (1 specimen) ; NTM W29620 (1 specimen) ; NTM W29621 (1 specimen) ; NTM W29622 (1 specimen) ; NTM W29623 (1 specimen) , collection details as for holotype, except for NTM W29622 collected on July 27, 2017 . AM collection: AM W.50329 (1 specimen, parapodia prepared for SEM), 1 piece of tissue AM W.50116 sequenced; AM W.50330 (1 specimen, parapodia prepared for SEM), 1 piece of tissue AM W.50118 sequenced; AM W.50331 (1 specimen) , 1 piece of tissue AM W.50120 sequenced; AM W.50332 (1 specimen) , 1 piece of tissue AM W.50122 sequenced, collection details as for holotype .
Comparative material examined. Marphysa bulla Liu, Hutchings & Kupriyanova, 2018 , holotype AM W.49124; M. hongkongensa Wang, Zhang & Qiu, 2018 , paratypes AM W.50930–50941; M. maxidenticulata Liu, Hutchings & Kupriyanova, 2018 , holotype, AM W.49113; M. mossambica ( Peters, 1854) , non-types, 10+( NTM W25488), Dumangas, Iloilo, Philippines, coll. J. Monteros-Receinte, 7 May 2010 .
Description. (based on holotype, with variation in parentheses for paratypes, unless otherwise noted)
Live worms anteriorly iridescent, pinkish, posteriorly cream-coloured with contrasting red branchial filaments; mud-filled gut visible through body wall from anterior-mid body, especially prominent posteriorly ( Fig. 1B View FIGURE 1 ). Immature male and females indistinguishable; gravid hatchery specimens with whitish pigmentation inside body wall. Preserved specimens 225 (201 – 308+) chaetigers, 99 mm (95 – 165+) long, 2.6 mm (2.6 – 3.7) width at chaetiger 10, excluding parapodia. Body elongate and tapered gradually at both ends, anteriorly not flattened ( Fig. 1C View FIGURE 1 ).
Prostomium rounded anteriorly with two dorsoventrally flattened buccal lips and an anterior notch between them ( Fig. 1D, E View FIGURE 1 ). Two palps and three antennae slender and tapering, each with short palpophores, arranged in an arc on posterior margin of prostomium. Antennae more or less smooth, of equal length, slightly longer than palps, about 1.5 times longer than prostomium. Eyes present, 1 pair, very faint, located at posterior base between palps and lateral antennae ( Fig. 1E View FIGURE 1 ; not visible in larger paratypes). First peristomial ring about 2.5 times longer than second one dorsally, with shallow notch on anterior margin, ventrally.
Maxillary apparatus ( Fig. 2A, B View FIGURE 2 ) not everted in holotype or paratypes; dissected out from the holotype. Maxillae with carriers and four paired elements and one single one, formula as follows: MF = 1+1, 4+5, (4)5+0, 3(4)+5(6), 1+1 (based on holotype and 2 paratypes AM W.29619, AM W.29621). MI approximately 2.5–2.7 times longer than maxillary carrier, rectangular anteriorly, triangular posteriorly, with a pair of oval wings situated at posterolateral margins. MI forceps-like, without attachment lamellae; well developed, sub-right-angle falchal arch. Closing system approximately 6 times shorter than MI. Ligament between MI and MII rectangular, dark. MII wide, without at- tachment lamella, teeth triangular, recurved, and distributed in less than half of plate length. Ligament between MII and MIII absent (or not sclerotized). MIII, single, slightly shorter than right MIV, curved forming part of distal arc; with recurved equal-sized triangular teeth; short attachment lamella in centre at base, elongate, generally dark, but lighter coloured posteriorly. Left MIV short (half the size of right MIV) with wide, rounded base, left 2 teeth longer than right-most one; attachment lamella dark, semi-circular. Right MIV with teeth triangular, recurved, decreasing in size posteriorly; attachment lamella large, wide, best developed centrally. MV, paired, rectangular (as long as wide), with a broad cutting edge, and no clearly-defined teeth (but following tradition to score as 1+1). Mandibles ( Fig. 2C View FIGURE 2 ) dark, with fine longitudinal stripe near inner edge visible dorsally; slightly shorter than MI plus carriers; cutting plates whitish, without distinct growth rings.
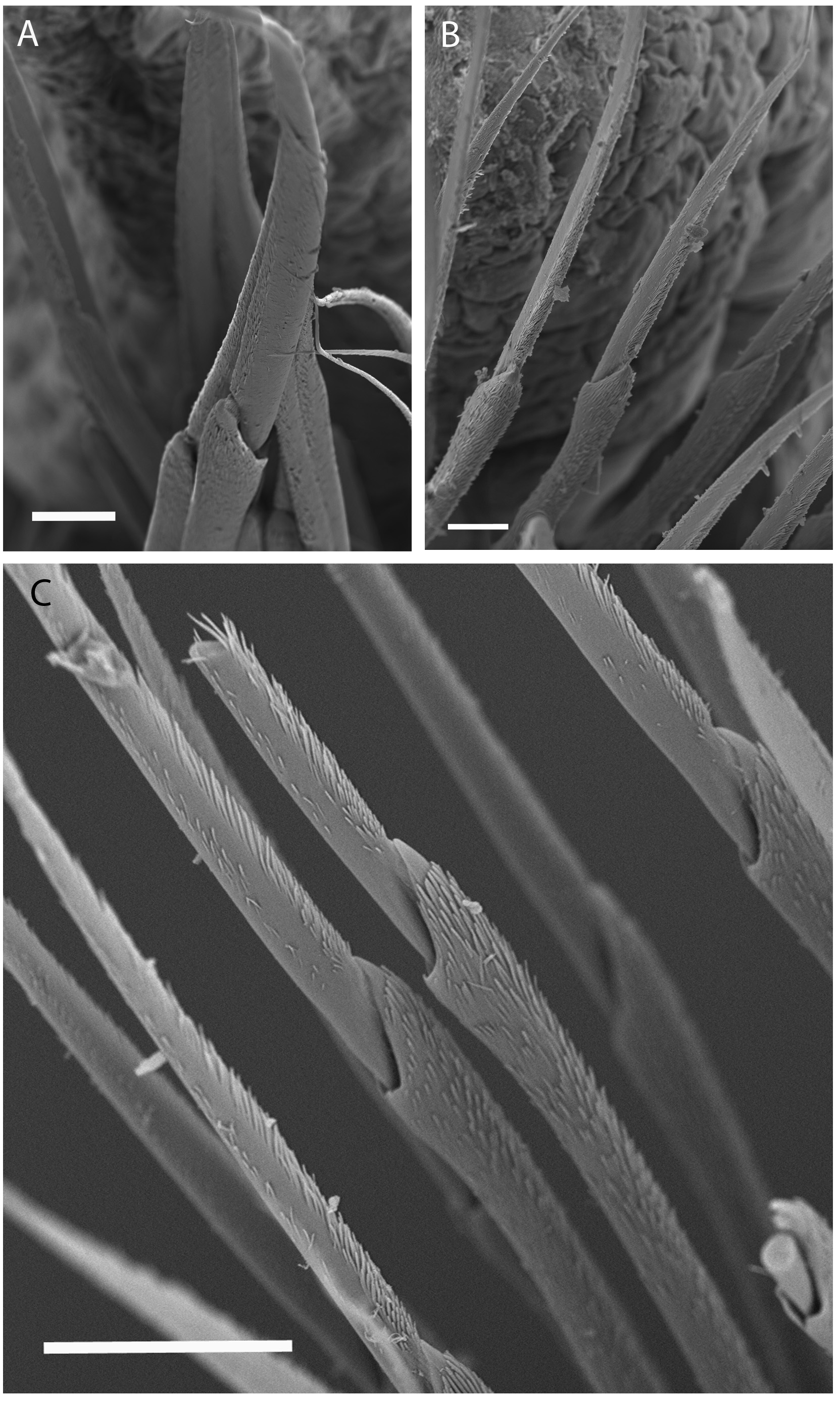
First few parapodia located below middle line of body wall, but gradually positioned dorsally to about midline in subsequent segments. Notopodial cirri ( Fig. 2 View FIGURE 2 D–I) slender, tapering, anterior ones faintly annulated ( Fig. 3A View FIGURE 3 ), extending laterally slightly further than both post-chaetal lobe and ventral cirri; extending slightly further than chaetal lobes in mid-body chaetigers; and similar lateral extension as post-chaetal lobes in posterior chaetigers, except for posterior-most ( Fig. 2I View FIGURE 2 ). Chaetal lobes comprising a low pre-chaetal lip and a tongue-like post-chaetal lobe. Ventral cirri ( Fig. 2 View FIGURE 2 D–I) bluntly conical, bases slightly expanded, first few exceeding lengths of post-chaetal lobes, thereafter slightly shorter than length of post-chaetal lobes. Branchiae pectinate ( Fig. 2 View FIGURE 2 E–I), commencing from chaetiger 19 (16–20) and continuing to near end; number filaments increasing from 1 or 2 anteriorly to 6–7 in mid-body, decreasing to 1 or 2 in last several chaetigers. Branchiae strongly wrinkled and vascularised at base where best developed ( Figs 2G View FIGURE 2 , 3 View FIGURE 3 B–E).
Aciculae black with paler blunt tips, approximately 4 per parapodium in anterior chaetigers, 2 or 3 per parapodium in middle chaetigers, and 1 per parapodium in posterior chaetigers ( Fig. 2 View FIGURE 2 D–I). Supra-acicular chaetae with limbate capillaries and pectinates; capillaries present from first chaetiger to near pygidium, numbering up to 20 in anterior chaetigers ( Fig. 4 View FIGURE 4 A–D).
Pectinate chaetae commencing from first few chaetigers to near end, three types identified: isodont-narrowslender (INS; Fig. 5 View FIGURE 5 A–C) having 8–19 long teeth, isodont-wide-slender and short (IWS; Fig. 5 View FIGURE 5 G–I) having 22–28 short teeth, and anodont-narrow-thick (ANT; Fig. 5 View FIGURE 5 D–F) having 5–10 short teeth, with numbers and distribution detailed in Table 3 View TABLE 3 . IWS pectinates replacing INS pectinates posteriorly; ANT pectinates absent anteriorly, appearing from anterior-mid body. Pectinate chaetae within each varying along the body: in general, the number of teeth, width and curvature increasing posteriorly. Note that our ANT pectinates approaching the isodont condition of other authors, with the lateral teeth sometimes significantly longer than central ones ( Fig. 5D, E View FIGURE 5 ).
Subacicular chaetae compound spinigers and subacicular hooks ( Fig. 2 View FIGURE 2 F–I). Compound spinigers commencing from first chaetiger to near pygidium, with long, tapered blade bearing bilateral fine serrations and serrated shaft ( Fig. 6 View FIGURE 6 A–C). Subacicular hooks amber to black, commencing from anterior chaetiger 30–38 (range for all types) to near end and inferior to bundle of spinigers, one per parapodium; slightly thinner than aciculae; subacicular hooks unidentate ( Fig. 2G View FIGURE 2 , inset-I).
Pygidium round, dorsally positioned, with 2 pairs of tapering pygidial cirri attached at ventral edge, dorsal pair two times length of ventral pair, approximately half of pygidial diameter ( Fig. 1F View FIGURE 1 ).
Taxonomical remarks. The new species is most similar morphologically to M. hongkongensa Wang, Zhang & Qiu, 2018 . However, there are some subtle, but distinct differences between the two species, including the presence of a pair of faint eyes in the new species, which are not described or illustrated for M. hongkongensa ; in the form of the pectinate chaetae, especially the isodont-wide-slender pectinates of mid and posterior parapodia, which have 22–28 teeth in the new species, but 15–23 in M. hongkongensa ; and the ANT pectinates which have 5–10 teeth in the new species, but 7–13 in M. hongkongensa . Further, the new species has only unidentate subacicular hooks, whereas M. hongkongensa also has bidentate hooks. The new species also differs in the length of the mandibles, which are slightly shorter than maxillae I plus carriers in the new species, but slightly longer in M. hongkongensa ; the palps which are subequal in length to the antennae in the new species, but equal in length in M. hongkongensa ; and the fewer number (6 or 7) of branchial filaments in the new species compared to 10 in M. hongkongensa .
The new species is also similar to M. bulla and M. maxidenticulata , both from the Yellow Sea, China. It can be distinguished from M. bulla by the earlier start of branchiae (chaetiger 16–20): versus 36–39 in M. bulla , by the subacicular hooks which are black proximally and pale distally in the new species, but amber-coloured in M. bulla . From M. maxidenticulata , the new species can be distinguished by the earlier start of branchiae (chaetiger 16–20): versus 28–41 in M. maxidenticulata , by the greater maximum number of branchial filaments (6 or 7) versus 3 in M. maxidenticulata , as well as by the subacicular hooks which occur singly and are black proximally, but pale distally in the new species, but are 1 or 2 per parapodium and are pale yellow throughout in M. maxidenticulata .
The form and distribution of pectinate chaetae has proved to be useful character for differentiating Marphysa species; however, we caution on using this character in a formulaic way for species discrimination. Pectinate chaetae are difficult to classify into groups because each of the class categories shows within-category variation along the body: in general, the number of teeth, width and curvature increases posteriorly in each category of pectinate chaetae. Therefore, despite the best intentions of previous workers to standardise this suite of characters based on teeth length, evenness, and chaetae thickness, its usefulness in separating species may be limited. For example, de- termining whether there are three or four different types of pectinates is very difficult without an exhaustive examination of multiple parapodia along the body of multiple specimens. Note that we have interpreted the new species to have three types of pectinates, whereas the types of the closely similar M. hongkongensa were said to have four types: the additional fourth type, median-toothed anodonts with 13–20 teeth, probably is equivalent to our INS-type pectinates with 8–19 teeth, which occur in the middle body chaetigers in low numbers. Therefore, we recommend the use of other characters in addition to pectinate chaetae to facilitate accurate species identification (see Key).
Etymology. The new species is named for the province of Iloilo, Panay Island, in the Western Visayas region of the Philippines, where numerous fish ponds and coastal mangrove wetlands are home to the new species.
Type locality. Tigbauan, Iloilo Province, Philippines.
Distribution and habitat. The type specimens were selected from hatchery brood stock collected from soft, fine, and muddy sediment in aquaculture tanks (0.20 m ²) at the Marine Annelids Hatchery of SEAFDEC/AQD, Tigbauan, Iloilo. The new species also occurs in brackish water milkfish ponds of the Dumangas Brackishwater Station, the site of collection of the egg-bearing jelly cocoons introduced into the Marine Annelids Hatchery, as well as the brackish water milkfish ponds in the northern part of Iloilo Province. Jelly cocoons were also present on occasions in the low tide zone of mangrove areas adjacent the discharge/intake points of the Dumangas ponds (MAM, pers. obs.), which may be from the new species (see below).
Reproduction and biology. At the Marine Annelid Hatchery, the larvae were grown for one month in the nursery, and thereafter the juveniles were transferred to grow-out tanks for four to five months. After five months, juveniles became sexually mature and spawned. Females produce jelly cocoons into which eggs are laid. The cocoons were found attached to the entrance of the adult burrow ( Fig. 1A View FIGURE 1 ).
At least three species of Marphysa appear to be present in the coastal mangrove areas of Iloilo Province. The jelly cocoon-making M. iloiloensis n. sp. and M. gravelyi , and the larger (perhaps cocoon-making – see Discussion) species, M. mossambica . Pillai (1962, 1965) reported that M. gravelyi were considered a nuisance to milkfish farmers in the 1960’s, as startled fish fry could dart into the jelly cocoons and become trapped. Nowadays the jelly cocoon-making species are viewed more favourably. Indeed, they are highly valued as a component of the maturation diet for tiger prawn brood stock in the shrimp aquaculture industry of the region. Marphysa species in general are widely collected by local people for fishing bait (MAM, pers. obs.).
| NTM |
Northern Territory Museum of Arts and Sciences |
| AM |
Australian Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.