Spio arndti, Bick, Andreas & Bastrop, Ralf, 2011
|
publication ID |
https://doi.org/ 10.5281/zenodo.277166 |
|
DOI |
https://doi.org/10.5281/zenodo.5697055 |
|
persistent identifier |
https://treatment.plazi.org/id/03CF3732-FFCC-FFC8-39E8-FE5384D53224 |
|
treatment provided by |
Plazi |
|
scientific name |
Spio arndti |
| status |
sp. nov. |
Spio arndti View in CoL sp. nov.
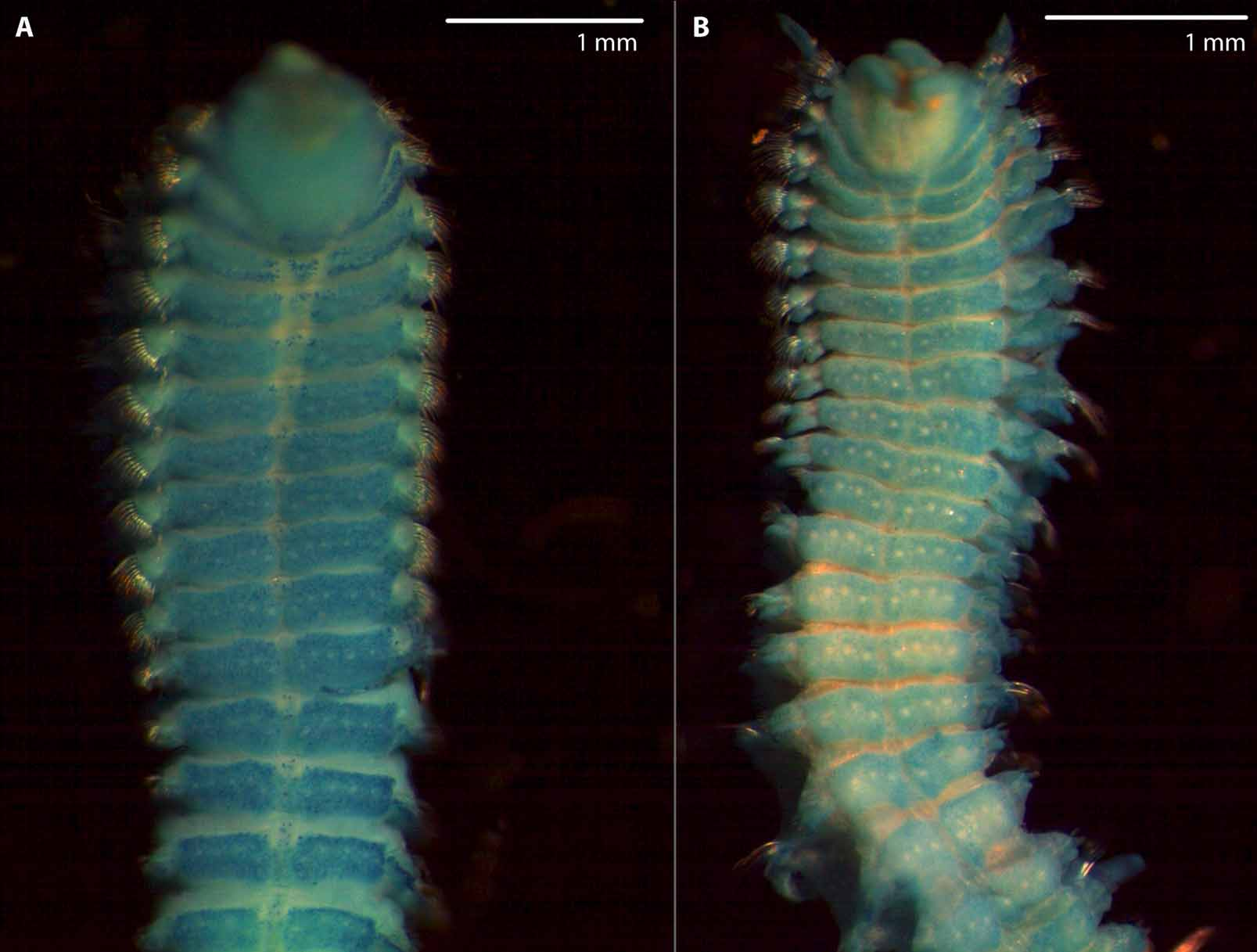
( Figs 7 View FIGURE 7 B, D, 8B, 9, 10)
Spio filicornis ( O.F. Müller, 1776) View in CoL . – Bick & Gosselck, 1985: 242–243, plate XXXII, fig. 3. Spio cf. filicornis (Baltic Sea) Bick et al., 2010: 168 View in CoL –171, figs. 4, 9e.
Holotype. Baltic Sea: 54° 30.154N, 10° 01.733E, 26.09.2009, 15.7 m ( ZSRO P- 2061).
Paratypes. Baltic Sea: 54° 02.168N, 11° 05.855E, 24.8.2005, 15 m, 1 specimen ( ZSRO P- 2042); 54° 43.784N, 10° 49.859E, 23.9.2005, 17.8 m, 1 specimen ( ZSRO P- 2043); 54° 33.131N, 10° 52.904E, 15.09.2005, 17 m, 8 specimens ( ZSRO P- 2050); 54° 30.154N, 10° 01.733E, 26.09.2009, 15.7 m, 4 specimens ( ZSRO P- 2051).
Non– type material. Baltic Sea: 54° 18.405N, 11° 12.030E, 15.10.1997, 16 m, 4 specimens ( ZSRO P- 409); 54° 20.10N, 12° 04.58E, 28.08.1997, 19 m, 4 specimens ( ZSRO P- 410); 54°10N, 11°10E, 29.07.1980, 22 m, 3 specimens ( ZSRO P- 1363); 54° 23.18N, 11° 57.48E, 27.10.1995, 17 m, 1 specimen ( ZSRO P- 1748); 54° 35.993N, 10° 27.019E, 27.10.2007, 18.1 m, 1 specimen ( ZSRO P- 2049); 54° 34,131N, 10° 50,557E, 15.09.2005, 20 m, 4 specimens ( ZSRO P- 2052); 54° 18.50N, 12° 07.50E, 25.09.2001, 18 m, 1 specimen ( ZSRO P- 2053); 54° 04.490N, 11° 24.350E, 15.11.2003, 16 m, 1 specimen ( SBRO P- 2557); 54° 10.550N, 11° 45.000E, 20.08.2002, 17 m, 2 specimens ( SBRO P- 2568); 54° 10.100N, 11° 45.000E, 15.09.2004, 5 m, 1 specimen ( SBRO P- 3493); 54° 28.800N, 12° 15.800E, 26.09.2004, 24 m, 2 specimens ( SBRO P- 3523); 54° 10.550N, 11° 45.000E, 0 3.02.2004, 19.5 m, 1 specimen ( SBRO P- 3541); 54° 31.012N, 12° 18.584E, 12.03.2004, 22 m, 1 specimen ( SBRO P- 3542); 54°27.93'N, 12°17.750'E, 18.02.2004, 18 m, 1 specimen ( SBRO P- 3543); 54° 04,490N, 11° 24,350E, 0 3.11.2005, 15.5 m, 1 specimen ( SBRO P- 4762).
Diagnosis. Anterior part of prostomium bluntly rounded, posterior part with high, narrow, keel-shaped elevation; anterior part of prostomium with light brown and peristomium with dark brown pigmentation; palps with light brown pigment along the margin of the food groove; nuchal organs with two pairs of more or less straight ciliated bands before 2nd tcb on chaetiger 3; branchiae of chaetiger 1 slightly shorter and narrower than those on following chaetigers, absent on last four to five chaetigers; double-paired metameric dorsal ciliated organs present between transverse ciliated bands (tcb) of two consecutive segments, starting between tcb of chaetigers 3 and 4, i.e., after second tcb, continuing up to tcb of chaetigers 15–19, i.e., up to tcb 14–18; notopodial postchaetal lamellae on last abranchiate chaetigers elongated cirriform; neuropodial postchaetal lamellae on median chaetigers narrow but distinctly elongated dorsally.
Description. Holotype complete specimen (without palps) with 48 chaetigers, about 9 mm in length and 0.8 mm wide. Other examined specimens with 32 to 57 chaetigers, 9 to 17 mm in length and 0.7 to 1.3 mm in width.
Anterior part of prostomium anteriorly rounded, very rarely with median incision ( Figs 7 View FIGURE 7 B, 9A); posterior part of prostomium with high, narrow, keel-shaped elevation, beginning in front of anterior pair of eyes and terminating on chaetiger 1 ( Fig. 7 View FIGURE 7 D); indistinct transverse depression between anterior and posterior part of prostomium; 2 pairs of black eyes arranged in trapezoid, anterior pair crescent-shaped or oval, widely spaced, posterior pair oval or round, closely spaced (dark brown spots of pigment between anterior and posterior eyes easily to be mistaken as a third pair of eyes) ( Figs 7 View FIGURE 7 B, D); prostomium distinctly separated from peristomium by furrow (Fig. 9A).
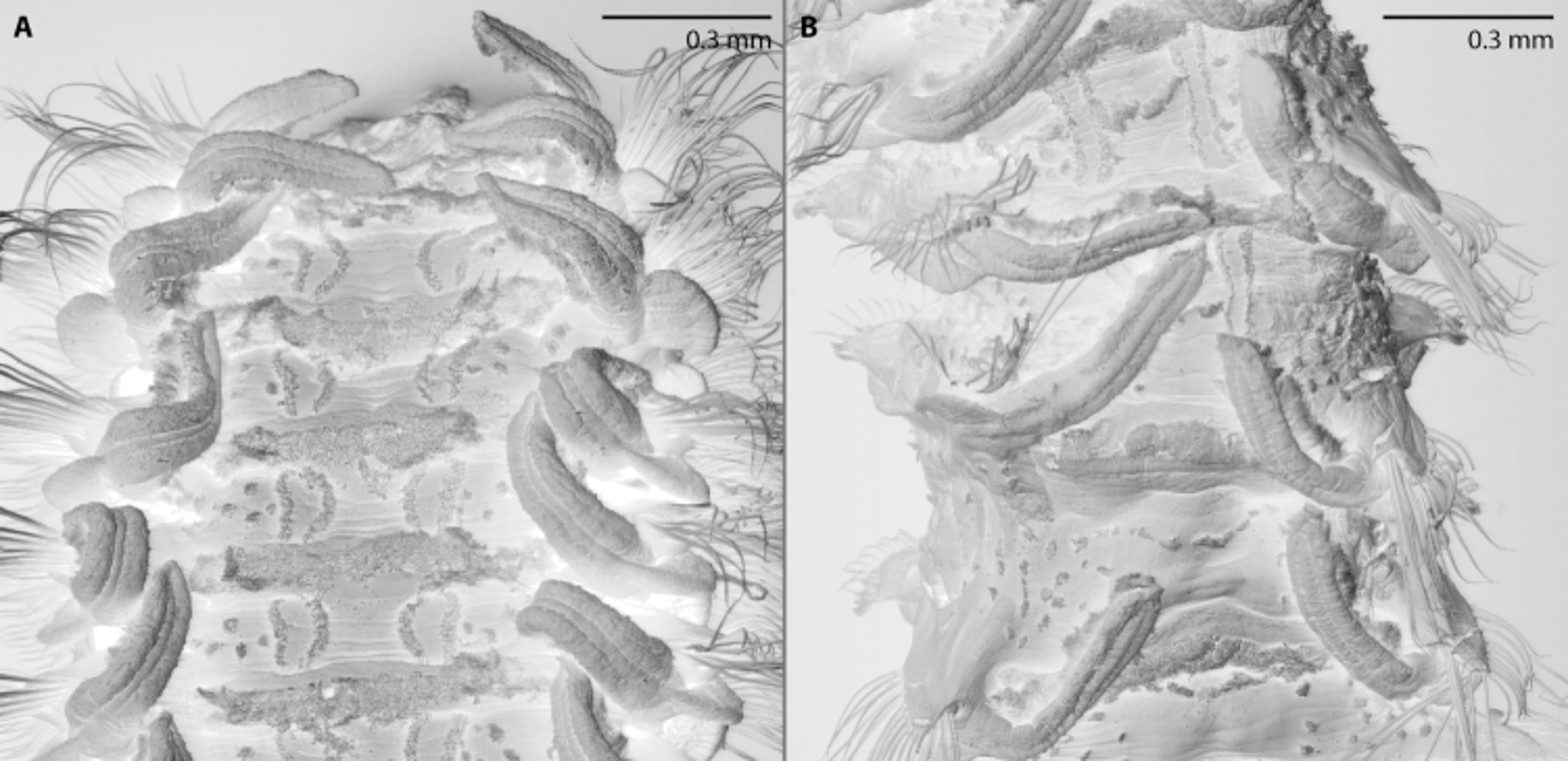
Nuchal organs with two pairs of more or less straight ciliated bands up to second tcb on chaetiger 3; median pair of ciliated bands interrupted by 1st tcb on chaetiger 2: anterior parts of median ciliated bands from just posterior to prostomium to 1st tcb, posterior parts of median ciliated bands posterior to 1st tcb; pair of lateral ciliated band outwards curved in range of first tcb; median and lateral ciliated bands not connected in front of second tcb (Fig. 9A); metameric dorsal ciliated organs as double-paired ciliated bands after second tcb (Fig. 9A), usually up to 16th tcb on chaetiger 17 (maximum extension ranges from 15th tcb on chaetiger 16 to 19th tcb on chaetiger 20) accompanied by 3 to 4 ciliated tufts ( Fig. 10 View FIGURE 10 ). Branchiae from chaetiger 1, continuing to almost the end of body, only last 4 to 5 chaetigers without branchiae (neotype: 5 chaetigers without branchiae); branchiae on first chaetiger slightly shorter and narrower than those on following chaetigers (Fig. 9A), reaching midline dorsally and touching each other on some anterior chaetigers, becoming thinner and shorter posteriorly: last or last two branchiae distinctly shorter than notopodial postchaetal lamellae (Fig. 9B); branchiae with narrow base, tapering distally, with cilia on inner and a longitudinal elevation on outer side ( Fig. 10 View FIGURE 10 ); branchiae on anterior chaetigers basally fused with notopodial postchaetal lamellae, separated from lamellae in posterior chaetigers (Figs 9C–H).
First notopodium shifted dorsally. Notopodial postchaetal lamellae on anterior chaetigers oval, base of postchaetal lamellae longer than transverse row of corresponding chaetae in most anterior chaetigers; notopodial postchaetal lamellae becoming smaller and rounded on middle chaetigers and subtriangular on posterior chaetigers, upright and cirriform on very last abranchiate chaetigers (Figs 9B–H). Neuropodial postchaetal lamellae in anterior chaetigers rounded, becoming smaller and elongated from about chaetiger 12 to 14, oval in posterior chaetigers (Figs 9B–H). Notopodial and neuropodial postchaetal lamellae may touch each other or are even interconnected on some anterior or medium chaetigers (Figs 9E, F).
Notopodial chaetae all capillaries with narrow sheaths; capillaries of anterior chaetigers arranged in two rows: chaetae of anterior row short, broad, distinctly granulated; chaetae of posterior row longer, thinner, lacking granulations; additional superior fascicle of very long, thin capillaries without granulations; capillaries of posterior chaetigers arranged in irregular rows, thin, non-granulated, of different length. Neuropodia with rows of capillaries and hooded hooks as well as an inferior fascicle of capillaries; capillaries of anterior neuropodia arranged in two rows, similar to notochaetae (Fig. 9J); posterior row replaced by single row of about 8 (5 to 9, and 5 to 6 in holotype) hooded hooks from chaetiger 11; on posteriormost chaetigers a third row of very thin alimbate finely granulated capillaries (Fig. 9I); hooks not narrowed subdistally, with a short hood, bidentate, main fang and apical tooth well developed (Fig. 9K); inferior fascicle with 2 to 5 long, thin, limbate capillaries without granulations from chaetiger 1, replaced by 2 to 3 sabre chaetae from about chaetiger 20–24, each distally granulated.
Pygidium with four anal cirri; dorsal pair slightly longer than ventral pair or of same size; dorsal pair more widely spaced than ventral pair; anus terminal.
Pigmentation. Palps with light brown pigmentation along food grooves, pigment stable in formalin- and alcohol-preserved specimens; preserved specimens with brown pigment on anterior part of body as follows: peristomium dark brown, especially on median (adjacent to prostomium) and posterior margin ( Figs 7 View FIGURE 7 B, 9A); pigmented patches in front of tcbs dorsolaterally from chaetiger 2 to chaetiger 15 at maximum (chaetiger 2 to 10 in neotype), sometimes also slightly pigmented behind tcbs ( Figs 7 View FIGURE 7 B, 9A); brown pigment between and around noto- and neuropodial lamellae, particularly distinct on first two or three chaetigers (distinct pigmentation present on chaetigers 1 to 9 in holotype) ( Fig. 7 View FIGURE 7 D); distinct brown spots ventrolaterally at anterior margin of chaetiger 2 to about chaetiger 9 or 10 (chaetiger 15 at maximum, and chaetiger 2 to 10 in holotype); anterior and posterior margin of branchial base from chaetiger 1 to about 8 with brown pigment (chaetiger 1 to 4 in holotype); prostomium from anterior margin to transverse depression with light brown pigment, but pigment sometimes completely absent; usually pigment patches or pigment stripes close to eyes; anterior margin of prostomium and median margins of peristomium with light brown pigment.
Methyl green staining pattern. Anterior part of prostomium and peristomium intensively stained; margins of postchaetal lamellae and branchiae of anterior and medium chaetigers intensively stained; in posterior chaetigers postchaetal lamellae and branchiae as well as anal cirri completely deep blue after staining.
About 10 minutes after specimens transferred from methyl green solution into distilled water again, 1 to 4 (rarely 5) pairs of white dots against bluish background become visible ventrally on anterior chaetigers ( Fig. 8 View FIGURE 8 B). First pair of dots usually visible on chaetiger 4, about 4 pairs are present from chaetiger 8 to chaetiger 15–17, diminishing continuously to 1 pair on about chaetigers 25 (or rarely chaetiger 30; up to chaetiger 24 in holotype). Following pattern of white dots were found on chaetigers 4–24 on the specimens investigated in detail (n=9, data for the holotype in parentheses): chaetiger 4–5: 1 pair of white dot (1 in holotype), 6: 2 (2), 7: 3 (3), 8–15: 4 (4), 16–17: 4 (3), 18: 3 (3), 19–22: 2 (1), 16: 3 (4), 17–19: 2 (3), 20–22: 2 (1), 23–24: 1 (1). These dots are arranged in a row but the median pair is slightly shifted posteriorly. As mentioned above they correspond obviously to ventral openings shown for S. filicornis (Fig. 5) (see discussion).
Biology. Spio arndti sp. nov. was found in sandy or pebbly sediments in subtidal regions. It was usually found in small numbers (fewer than 10 specimens per square metre).
FIGURE 9. Spio arndti sp. nov.: A. Anterior end, dorsal view. B. Posterior end, dorsolateral view; pygidial cirri broken off. C– H. Parapodium from chaetiger 1, 10, 11, 20, 18th last, and 9th last, all anterior view. I. Posterior neurochaeta from parapodium of the 18th last chaetiger. J. Anterior neurochaeta from parapodium of chaetiger 20. K. Neuropodial hooded hook from the same. — All from Kiel Bight (ZSRO-P2050); Scale: A, B 0.5 mm, C–H 0.1 mm, I–K 10 µm.
Etymology. The species is named after Professor Ernst Albert Arndt. He was a lecturer for zoology and marine biology at Rostock University and an expert for macrozoobenthos of the Baltic Sea. All three authors of this paper had been his students.
Geographical distribution. So far known only from the western Baltic Sea (type locality).
Remarks. Spio arndti sp. nov., is distinguished from all other Spio species by the posterior extension of the dorsal ciliated organs up to the 15th tcb on chaetiger 16, the number of posterior chaetigers (1 or 2) with branchiae shorter than the notopodial lamellae and the number of posterior abranchiate chaetigers (four to five), the pigmented spots dorsolaterally in front of the tcbs on anterior chaetigers, and the distinct pattern of white dots with up to 4 pairs of dots ventrally.
Closely related species are S. filicornis , S. malmgreni and S. readi . Spio arndti sp. nov. differs from S. filicornis in the extension of the dorsal ciliated organ (up to chaetiger 16 instead of chaetiger 11), the shape of the posteriormost notopodial lamellae (cirriform instead of lobe-like), the number of abranchiate chaetigers (four to five as opposed to six to ten) and the pattern of pigmentation on the anterior end (dark brown pigment in front of the tcb dorsally instead of behind it ( Fig. 7 View FIGURE 7 A, B), and brown pigment spots ventrolaterally present rather than absent). In addition, the number of ventral white dots is lower in S. arndti sp. nov. compared to S. filicornis ( Fig. 8 View FIGURE 8 ). S. filicornis has a distinctly greater body width. Additional ciliary tufts adjacent to the dorsal ciliated organs are present only in S. arndti sp. nov.
Spio readi differs from S. arndti sp. nov. in having a narrow peristomium, large reddish brown pigment patches dorsally on peristomium and anterior chaetigers as well as 9 to 10 neuropodial hooded hooks; white dots or ventral glands are not described. Spio readi has non-granulated sabre chaetae with fine bristles rather than uniformly granulated ones occurring in S. arndti .
Specimens of S. arndti sp. nov. are morphologically very similar to S. malmgreni but S. malmgreni is larger than S. arndti sp. nov.: 2.1 mm wide, 40 mm in length and about 70 chaetigers. Moreover, the posterior extension of the dorsal ciliated organs differs, chaetiger 14 at maximum in S. malmgreni and usually chaetiger 16 in S. arndti sp. nov. The inferior neuropodial capillaries first appear on chaetiger 3 or 4 in S. malmgreni rather than on chaetiger 1 as in S. arndti sp. nov. Ventral subepidermal glands or white dots and additional ciliary tufts adjacent to the dorsal ciliated organs were not mentioned for S. malmgreni . On the other hand the shape of the nuchal organs, the pattern of pigmentation and the number of posterior abranchiate chaetigers are similar. As mentioned above, the description of S. malmgreni has to be enhanced particularly with regard to the pattern of pigmentation, the formation of ciliated bands and tufts on the dorsum, the number of hooded hooks and the occurrence of ventral glands. Intraspecific variations in S. arndti sp. nov. include the posterior extension of the metameric dorsal ciliated organ, the number of hooded hooks and the length of the first branchiae. The number of hooded hooks is size-dependent.
Smaller specimens (<1 mm wide) possess 6 hooks on average whereas larger specimens (> 1 mm wide) have about 8 or 9 hooks. Similar relationships have already been described by Bick et al. (2010) for several Spio species. A correlation of the posterior extension of the metameric ciliated organs with the size of the specimens could not be found. However, this extension ranges between chaetiger 15 to 19. The length of the first pair of branchiae varies between two-thirds as long as the second and slightly longer.
The shape of the nuchal organs on S. arndti sp. nov., (as S. cf. filicornis (Baltic Sea)) was misinterpreted by Bick et al. (2010). The lateral ciliated bands neither curve posteriorly nor go completely back to the first tcb. They are only outwards curved in the range of the first tcb on chaetiger 2, and the median ciliated bands are interrupted by the first tcb. The shape of the nuchal organ and the formation of the dorsal ciliated organs need to be paid greater attention to in future studies not only among Spio species but also in Spionidae generally (see Meißner & Blank 2009).
Spio arndti sp. nov. might not only occur in the Baltic Sea but this must be investigated.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.