Brycon Müller & Troschel
|
publication ID |
https://doi.org/10.5281/zenodo.257769 |
|
publication LSID |
lsid:zoobank.org:pub:F0EC0A87-B1EE-4B5C-8F53-77A7EEA75F3A |
|
DOI |
https://doi.org/10.5281/zenodo.6025286 |
|
persistent identifier |
https://treatment.plazi.org/id/C033D710-4F71-FFF6-4EA4-F9C4FB4AFA7A |
|
treatment provided by |
Plazi |
|
scientific name |
Brycon Müller & Troschel |
| status |
|
Genus Brycon Müller & Troschel View in CoL View at ENA
Brycon Müller & Troschel, 1844: 90 View in CoL . Type species: Brycon falcatus Müller & Troschel 1844 View in CoL . Type by subsequent designation ( Eigenmann, 1910: 430). Gender: masculine.
Chalcinopsis Kner, 1863: 226 . Type species: Chalcinopsis striatulus Kner, 1863 . Type by subsequent designation (original type designation not researched). Gender: feminine.
Megalobrycon Günther, 1869: 423 . Type species: Megalobrycon cephalus Günther, 1869 . Type by monotypy. Gender: masculine.
Catabasis Eigenmann & Norris, 1900: 358 . Type species: Catabasis acuminatus Eigenmann & Norris, 1900 . Type by original designation. Gender: feminine.
Othonophanes Eigenmann, 1903: 145 : Type species: Brycon labiatus Steindachner, 1879 View in CoL . Type by original designation. Gender: masculine.
Bryconodon Eigenmann, 1903: 146 . Type species: Brycon orthotaenia Günther, 1864 View in CoL . Type by original designation. Gender: masculine.
Holobrycon Eigenmann, 1909: 33 : Type species: Brycon pesu Müller & Troschel, 1845 View in CoL . Type by original designation. Gender: masculine.
Triurobrycon Eigenmann, 1909: 33 . Type species: Brycon lundii Lütken, 1875 View in CoL . Type by original designation. Gender: masculine.
Diagnosis. The genus Brycon can be diagnosed from all remaining characid genera, with the exception of Triportheus and Chilobrycon , by the possession of three rows of premaxillary teeth with a distinctive arrangement (see “Monophyly of Brycon ”, below, for more details). Brycon can be diagnosed from Triportheus for lacking the ventral keel formed by greatly developed coracoid bones extending from the isthmus to a little ahead of the anal-fin origin, characteristic of the latter genus, as well as possessing moderately-sized pectoral fins, reaching only the pelvic-fin origin (vs. pectoral fins elongated and pointed, their tips always beyond pelvic-fin origin in Triportheus ) and pelvic-fin originating approximately at the same level than dorsal-fin (vs. pelvic fins situated ahead of dorsalfin origin). Brycon can be distinguished from the closely related and monotypic genera Chilobrycon and Henochilus by possessing broad, bulky dentary and premaxillary teeth (vs. compressed, incisiform teeth; see Géry & de Rham, 1981; Castro et al., 2004), and from Henochilus by possessing three rows of premaxillary teeth (vs. two rows of premaxillary teeth in Henochilus ; see Castro et al., 2004). Brycon can be diagnosed from the somewhat similar-looking South American chalceid genus Chalceus by possessing relatively small scales, always more than 40 scales in lateral-line counts, and approximately of the same size across the body (vs. large scales, never more than 40 scales in lateral-line counts, and scales above lateral line considerably larger than those at lateral line or below it in Chalceus ), and a relatively long anal fin, with always more than 15 branched rays (vs. short anal fin, with 8–10, generally 9, branched anal-fin rays in Chalceus ). Additional remarks on the diagnosis and putative monophyly of Brycon are presented below.
Monophyly of Brycon . The monophyly of Brycon has been questioned for some time in the literature (e.g., Howes, 1982; Castro & Vari, 1990), and molecular evidence pointing its non-monophyly has recently emerged ( Abe et al., 2014; see “Intrarrelationships”, below). So far, no unique derived features were identified for the genus Brycon , and consequently, the monophyly of the genus still remains uncertain. Although a resolution on the question of the monophyly of the genus lies beyond the scope of the present contribution, comments on the distribution and variation of three relatively uncommon character-states that are usually employed for diagnosing the genus are presented below, with a view of helping future discussions on its putative monophyletic status.
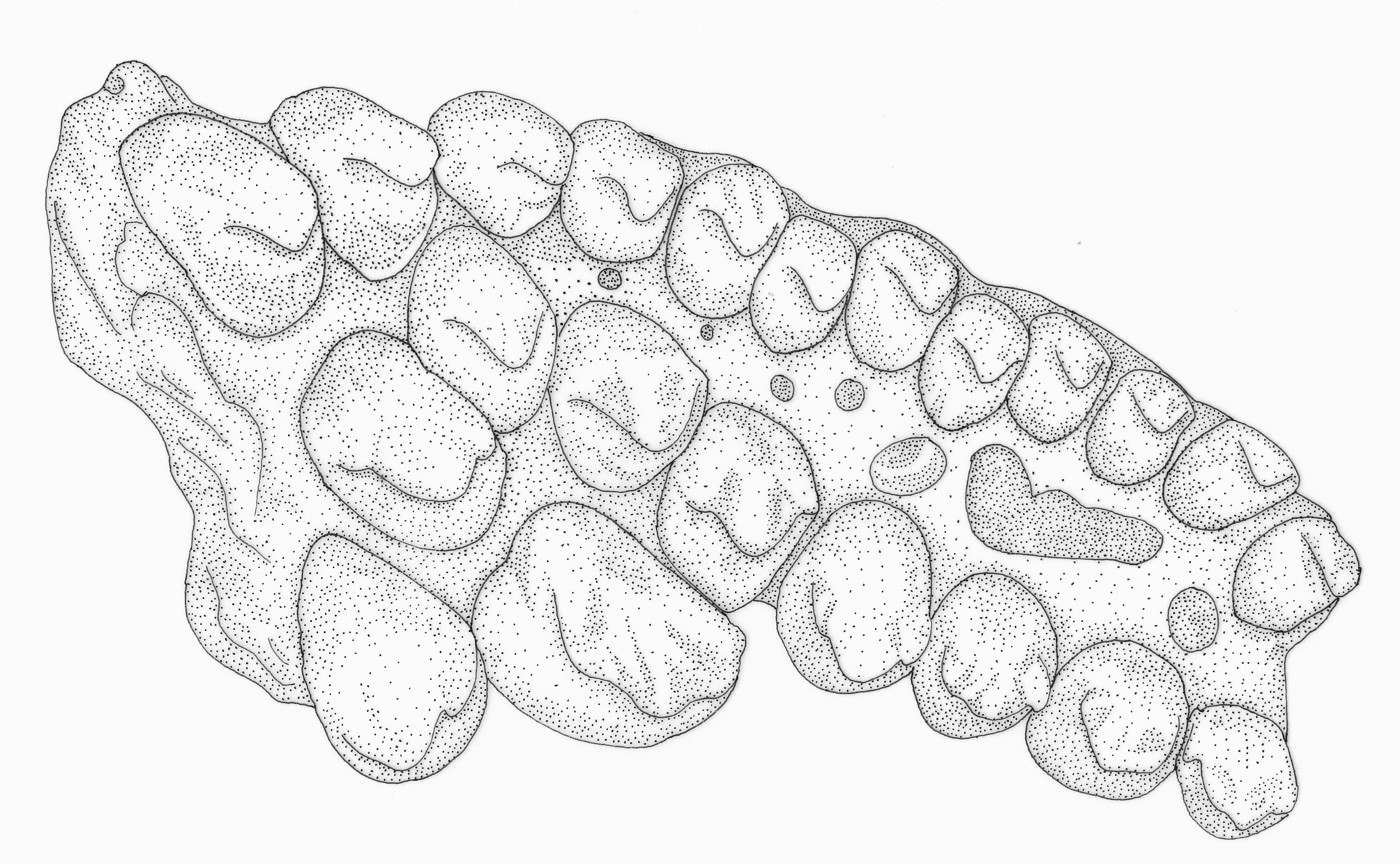
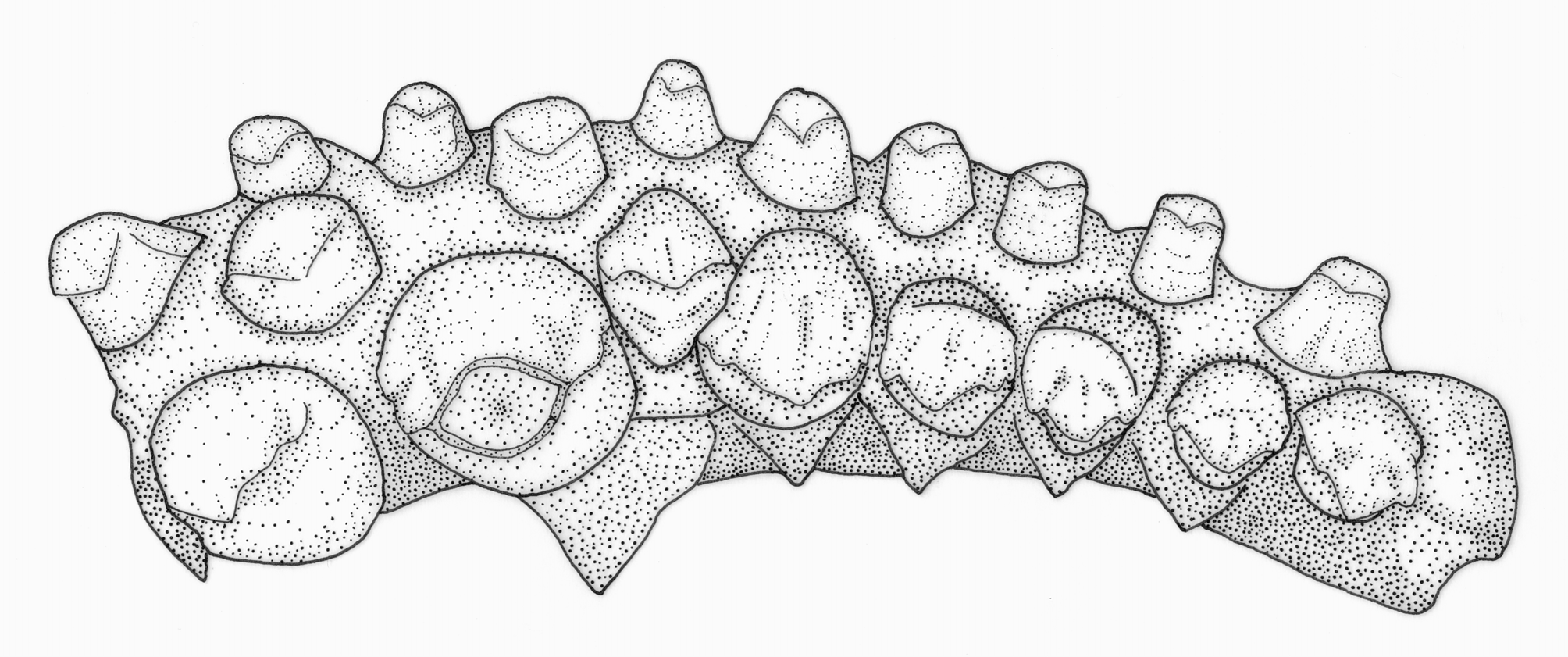
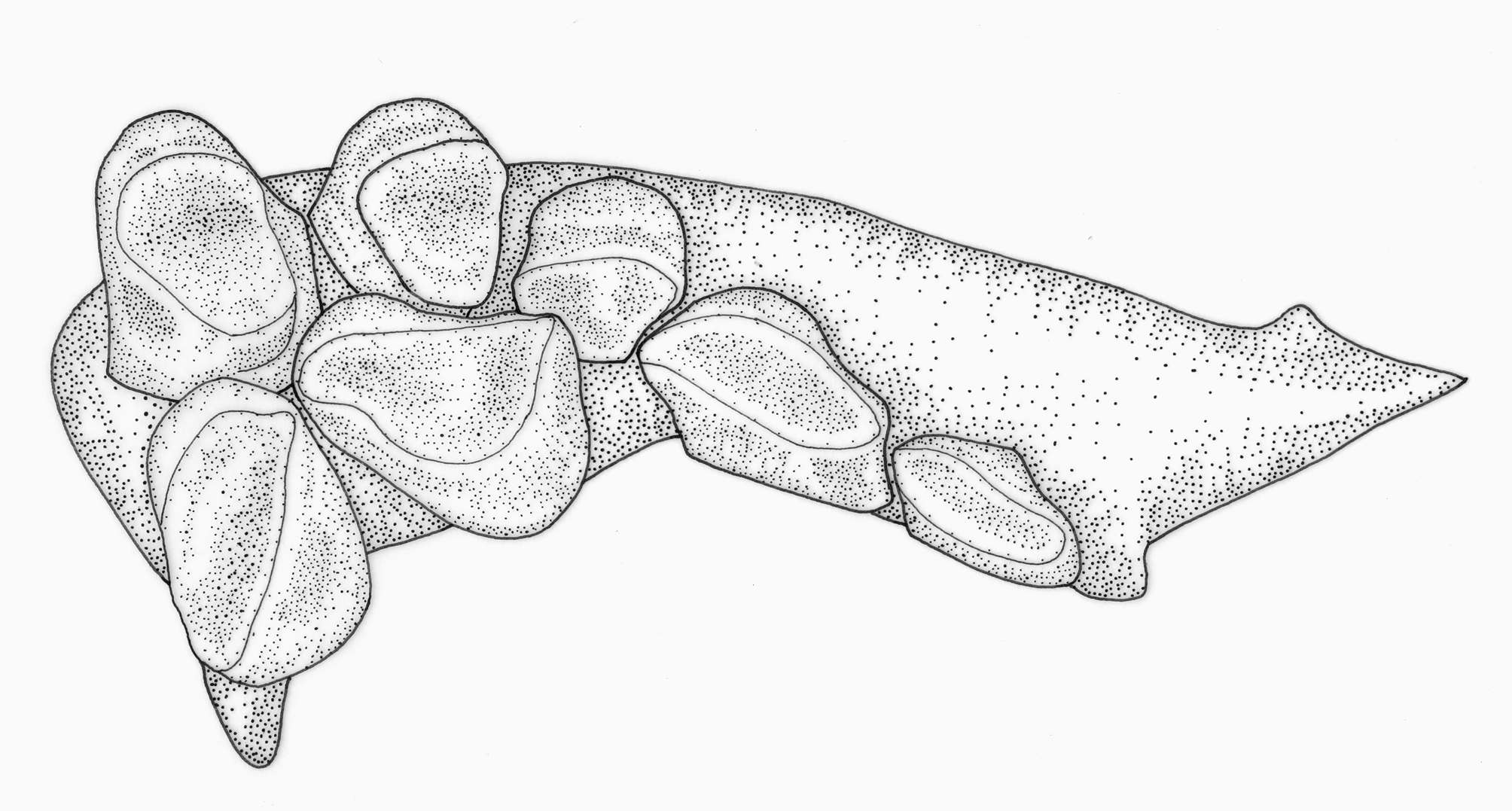
1. Three series of premaxillary teeth. Brycon species are unusual among characiforms in typically possessing three series of premaxillary teeth (four series of premaxillary teeth are present in Brycon dentex and some other trans-andean/middle American Brycon species; see Howes, 1982: 21, 23). An interpretation for the teeth arrangement in Brycon , first advanced by Böhlke (1958: 68), employed by Howes (1982: 2), and adopted herein, is to consider the outer series formed by small teeth as the first series, the pair of enlarged teeth near the symphysis of the premaxillary as the third series, and the middle-sized teeth situated at the inner (lingual), lateral border of the premaxillary and between the first, outer series and the inner, third series in the middle portion of the premaxillary as being the second series ( Figs. 1–2 View FIGURE 1 View FIGURE 2 ). Three premaxillary teeth rows are also found, among characids, in Bryconops alburnoides (e.g., Machado-Allison et al., 1993), Piabina , Creagrutus ( Vari & Harold, 2001: 12–13), Triportheus , and Chilobrycon , in the chalceid genus Chalceus and in the alestid Bryconaethiops ( Zanata & Vari, 2005: 32–33). The premaxillary teeth arrangement found in Creagrutus and Piabina , discussed in detail by Vari & Harold (2001: 12–13) and Ribeiro et al. (2004: 609), is very distinct and certainly not homologous to the one found in Brycon . We concur with Zanata & Toledo-Piza (2004: 105) and Zanata & Vari (2005: 32–33) in considering the condition found in Chalceus as non-homologous with the one found in Brycon . In Chalceus , the inner series, third series is formed by 5–10 teeth that extend from the premaxillary symphysis to the inner, lateral portions of the bone, while the second series is formed by two teeth situated between the first and third teeth rows ( Zanata & Toledo-Piza, 2004: 10). This is clearly a distinct condition from the one found in Brycon , where the pair of enlarged teeth does not seem to be closely associated with the smaller teeth situated in the inner, lateral portion of the premaxillary, which are instead distinctly aligned with the similar-sized teeth situated in the middle of the premaxillary, between the outer (first) and inner (third) premaxillary teeth rows ( Figs. 1–2 View FIGURE 1 View FIGURE 2 ; see also Howes, 1982: figs. 1, 2, 16, and 18). We have not examined any Bryconaethiops specimens, but the condition in this latter genus, as indicated by Zanata & Vari (2005: 33, 35, fig. 15; see also Roberts & Stewart, 1976: 274, fig. 3, and Teugels & Thys van den Audenaerde, 1990: 210, fig. 4) clearly does not correspond to the one found in Brycon and should also be considered as non-homologous. As for the condition presented by Triportheus and Chilobrycon , we concur with Zanata & Toledo-Piza (2004: 105) and Zanata & Vari (2005: 33) in considering it to be virtually undistinguishable from the condition found in Brycon (see Fig. 3 View FIGURE 3 ). Mirande (2010: 482) recovered the presence of three series of teeth on the premaxillary as one of the synapomorphies for Bryconinae (in his definition, including, besides Brycon , the genus Triportheus ), although when coding this character he disregarded the hypothesis of nonhomology between the condition found in these genera, on one hand, and the one found in Chalceus and Bryconaethiops , raised by Zanata & Toledo-Piza (2004) and Zanata & Vari (2005), which is also supported here. Another synapomorphy for Bryconinae involving the arrangement of the premaxillary teeth proposed by Mirande (2010: 482) was the “polymorphism of teeth on the inner premaxillary row… with two medial teeth somewhat larger and usually separated from remaining ones by a gap”. This corresponds to the presence of the inner, third series of teeth in Brycon , which Mirande (2010: 423) considered as also including the teeth present in the inner, lateral portions of the premaxillary. Interestingly, Mirande (2010: 471, 482) suggests that this character state is also present in Serrasalmidae , though the latter only present two teeth rows (with the exception of the piranhas, which possess a single row of premaxillary teeth; Machado-Allison, 1985: 22–25; Cione et al., 2009). Premaxillary teeth arrangement in basal Serrasalmidae is in fact remarkably similar to the one observed in Bryconinae not only in the possession of a pair of inner enlarged teeth near the premaxillary series (which corresponds, respectively, to what is generally considered to be part of the second series in Serrasalmidae , and the third series in Bryconinae ), but also because the outer, first teeth series of Serrasalmidae has a very similar arrangement to the second teeth series in Bryconinae (compare Figs. 1–2 View FIGURE 1 View FIGURE 2 with Fig. 4 View FIGURE 4 ; see also Jégu et al., 2003: 841, fig. 3c; Jégu et al., 2004: 131, fig. 7c). The difficulty in establishing homology among distict teeth rows in the premaxillary has been repeatedly discussed in the literature ( Howes, 1982: 2; Vari & Harold, 2001: 12; Zanata & Vari, 2005: 32–33; Mirande, 2010: 420–421). Mirande (2010: 420) suggested that developmental data could help in establishing the homologies of distinct premaxillary teeth rows, since he supposed that, while most of the premaxillary teeth developed intraosseously (i.e., in bone crypts), some of them developed extraosseouly, although there is some difficulty in establishing whether the development of some teeth are intraosseous or extraosseous in the group ( Trapani, 2001; Trapani et al., 2005). In our opinion, adequate hypothesis of homology between premaxilary teeth rows across the characifoms will ultimately depend on the identification and study of taxa presenting intermediate, transitional teeth arrangements between the main known types of teeth arrangements, whether using extant ( Ribeiro et al., 2004) or extinct ( Cione et al., 2009) examples.
2. Continuity of orbital ring. The series of ossifications surrounding the orbit is formed by six infraorbital bones, plus the supraorbital and the antorbital. The most common condition found among characiforms is to possess an incomplete orbital ring, either due to lack of contact between the sixth infraorbital with the supraorbital, and/or the supraorbital with the antorbital (e.g., Distichodontidae and Citharinidae ; Vari, 1979: 296–299; Serrasalmidae ; Machado-Allison, 1983: 163–164, 192, fig. 11; some Alestidae : Zanata & Vari, 2005: 6, fig. 1B; Acestrorhynchus ; Toledo-Piza, 2007: 713, fig. 14), or for the lack of supraorbital (e.g., Erythrinidae , Lebiasinidae , some Alestidae , and most Crenuchidae and Characidae ; e.g., Weitzman & Fink, 1983; Vari, 1995; Buckup, 1993, 1998; Malabarba & Weitzman, 2003; Zanata & Vari, 2005). In those cases, the orbit is partially delimited by the frontal bone. A complete, continuous orbital ring is reported among characiforms in some Hemiodontidae ( Roberts, 1974: 418–419), Hepsetidae ( Roberts, 1969; Vari, 1995), all Ctenoluciidae ( Roberts, 1969; Vari, 1995), some Alestidae ( Zanata & Vari, 2005: 9) , and, among Characidae , all Cynodontinae (e.g., Hydrolycus: Toledo-Piza, 2000 : fig. 2), Agoniates (e.g, Géry, 1962: fig. 3), Salminus (e.g., Roberts, 1969; Machado-Allison, 1983: 192, fig.
11), Triportheus , and most Brycon species. Lignobrycon appears to be variable on this regard; a cleared and stained specimen examined from the rio de Contas basin ( MZUSP 75230 View Materials ) possess a continuous orbital ring, while specimens from the rio de Una basin the sixth infraorbital does not contact the supraorbital ( Castro & Vari , 1990: fig. 2; A.M. Zanata, pers. comm.). As earlier remarked by Zanata (2000: 28), species of Brycon typically possess a continuous orbital ring, but there are some exceptions. Zanata (2000: 28) reported that Brycon henni (a transandean Brycon species widely distributed at the Río Cauca and other river drainages of northeastern Colombia; Maldonado-Ocampo et al., 2005), and B. coxeyi , lack a contact between the sixth infraorbital and the supraorbital bones. During the course of the present study, we noticed that Brycon stolzmanni also lack a contact between these bones as well. Since all these species also share the presence of a dark patch of dark pigmentation at the opercle, the lack of continuity in the orbital ring shared by them is probably best interpreted as a derived condition within the genus.
3. Inner symphyseal dentary teeth pair. The presence of a inner symphyseal teeth pair, slightly smaller than the teeth from the main dentary series situated immediately ahead, is an uncommon feature among characiforms, being present in the serrasalmids Colossoma , Piaractus , and Mylossoma ( Machado-Allison, 1986: 47) , Mylesinus paucisquamatus ( Jégu & Santos, 1988) , Tometes ( Jégu, Santos, Keith & Le Bail, 2002; Jégu, Santos & Belmont- Jégu, 2002), Utiaritichthys ( Jégu et al., 1992) , Myloplus ( Jégu et al., 2003; Jégu et al., 2004), Myleus (Jégu & Santos, 2002) , and in most alestids ( Zanata & Vari, 2005: 46–47). Among characids, it is reported only for the representatives of Bryconinae , i.e., Brycon , Triportheus ( Howes, 1982: 1–2), Henochilus (Castro et al., 2004: 501) , and Chilobrycon (Géry & de Rham, 1981: 10) . Contrary to Machado-Allison (1983: 174–175), Zanata & Toledo- Piza (2004: 105–106), Zanata & Vari (2005: 46–47), and Mirande (2010: 425), and as Uj (1990: 52–53), we prefer to consider the symphyseal teeth pair as a distinct, independent morphological character from the row of small inner row of conical teeth on the lingual crest of the teeth replacement trench. The symphyseal teeth pair has an intraosseous origin, being formed in an autonomous tooth fossa (cf. Roberts, 1967: 246, fig. 3; Machado-Allison, 1986: 47), unlike the inner conical teeth row, which has an extraosseous origin, being developed directly in the oral epithelium. Also, with the exception of the genera Brycon , Henochilus , Chilobrycon , and Chalceus , all remaining taxa displaying a symphyseal teeth lack an inner dentary teeth row. Mirande (2010: 425) states that Triportheus also possess a inner row of dentary teeth., apparently because he considered the small, distalmost teeth on the dentary as constituting the inner teeth row, since there is a overlap between the last teeth of the main dentary series and the first small teeth of dentary in the specimen of Triportheus nematurus he examined ( Mirande, 2010: 419, fig. 60). This overlap is, however, apparently atypical, as it was not observed in the material of Triportheus herein examined (see Comparative material examined at Appendix 1). Although it makes sense for developmental reasons to consider the small dentary teeth as being homologous to the inner teeth row rather than to the main dentary teeth row since they are developed extraosseously as well ( Trapani et al., 2005: 528; pers. obs.), the condition observed in Triportheus is not similar to the one found in Brycon , Chalceus , Brycon , and Henochilus , and it is questionable to consider it equivalent to the condition found in these latter genera. Contrary to the inner symphyseal dentary teeth, the presence of an inner dentary teeth row is a relatively widespread feature among characiforms. The presence of the inner symphyseal teeth is variable instraspecifically both in some alestids ( Zanata & Vari, 2005: 47) and serrasalmids (e.g., Tometes makue ; Jégu, Santos & Belmont-Jégu, 2002: 258, 260). In Brycon , the symphyseal teeth may be missing in large specimens of several of the large-sized species, such as Brycon vermelha , B. amazonicus , or B. falcatus , but is always present in juvenile and middle-sized specimens of all species.
Remarks. The genus Brycon was originally proposed by Müller & Troschel (1844) to include some species previously assigned to the genus Chalceus Cuvier ( C. macrolepidotus , C. amazonicus , and C. opalinus ), plus three species that were described in that contribution, Brycon falcatus , B. schomburgkii , and B. pesu . Müller & Troschel (1844), however, included in Brycon the type species of Chalceus , C. macrolepidotus , a mistake that prompted Valenciennes (in Cuvier & Valenciennes, 1850: 239) and Kner (1859: 9) into not recognizing Brycon as a valid genus. Günther (1864: 333–334) provided a new diagnosis for Brycon , and ever since this genus has been recognized as valid. Brycon , as currently understood, comprises seven synonyms, most of which were never consistently used in the literature. Two of these synonyms, Chalcinopsis Kner (1858: 223) and Othonophanes Eigenmann (1903: 145) were described having as type-species taxa from Central America or trans-andean South America, and are not further discussed here. Braum (1983b) discussed the synonym of Othonophanes with Brycon . A third synonym, Holobrycon Eigenmann (1909: 33) , was proposed to include Brycon pesu , a species not treated in the present contribution. See Howes (1982) for the synonymization of Holobrycon into Brycon .
Megalobrycon Günther (1869: 423) was erected having as its type species Megalobrycon cephalus . This genus was not explicitly compared with Brycon View in CoL at its original description, though it is clear from the description that its purported diagnosis from the latter genus was the lack of the inner symphyseal dentary teeth pair, behind the main teeth series. Cope (1872) included within Megalobrycon two species, M. melanopterum and M. erythropterum , without discussing the reasons that prompted him into including these species under this genus. Steindachner (1877: 26, 32) noticed that not all Brycon ferox View in CoL specimens he examined lacked the inner symphyseal teeth pair, and, on other hand, two specimens of Brycon melanopterus View in CoL from the NMW collection, a species supposedly lacking these teeth, possessed them. He consequently considered the lack of the pair of symphyseal teeth to be “accidental” or “abnomal”, but he still retained Megalobrycon as a sub-genus of Brycon View in CoL . In spite of Steindachner’s (1877) action, Miranda-Ribeiro (1902, 1905) continued to consider Megalobrycon a valid genus, listing all species previously assigned to the genus, and adding a new one, Megalobrycon piabanha (a synonym of Brycon insignis View in CoL ). Eigenmann (1910: 430), presumably following Steindachner’s (1877) remarks, synonymized Megalobrycon into Brycon View in CoL . Howes (1982: 19) examined the syntypes of Megalobrycon cephalus and noticed that although the larger specimen do in fact lack the inner pair of symphyseal dentary teeth, these are actually present in the smaller specimen. Lima & Castro (2000: 158) noticed the absence of the inner pair of symphyseal dentary teeth in the typical series of Brycon vermelha View in CoL . As remarked under the item “ Monophyly of Brycon View in CoL ”, above, the loss of the inner pair of symphyseal dentary teeth is a common feature of large-sized (generally> 300 mm SL) specimens of Brycon View in CoL , and, consequently, cannot be used as a diagnostic generic character.
The genus Catabasis was erected by Eigenmann & Norris (1900: 358), having as its type species C. acuminatus . Eigenmann & Norris (1900: 358) only compared it with Salminus View in CoL . Roberts (1969: 437–438) examined the holotype of Catabasis acuminatus and remarked that it actually resembled more closely Brycon acutus View in CoL (a synonym of Brycon alburnus View in CoL ; Howes, 1982) and Acestrorhamphus (= Oligosarcus View in CoL ) than Salminus View in CoL . Catabasis remained as an enigmatic taxon until Howes (1982: 4) sinonymized it with Brycon View in CoL , noticing that the absence of lateral cusps was the only significative distinction between the now B. acuminatus and its congeners. Further investigations on the subject revealed that Brycon acuminatus View in CoL should be considered a synonym of B. insignis View in CoL (see the item “Remarks” of Brycon insignis View in CoL , below).
Bryconodon Eigenmann (1903: 146) was proposed to include Brycon orthotaenia View in CoL , diagnosed by lacking the second teeth row on the dentary (an information stated on the original description of B. orthotaenia View in CoL ; Günther, 1864: 335). A little later, Eigenmann himself recognized Bryconodon as a mere synonym of Brycon ( Eigenmann, 1910: 430) View in CoL . As noticed in the description of Brycon orthotaenia View in CoL , below, contrary to what was stated by Günther (1864), this species does indeed possess the inner row of small teeth on the distal portion of the dentary.
Triurobrycon Eigenmann (1909: 33) was described having as its type-species Brycon lundii View in CoL (= Brycon orthotaenia View in CoL , see item “Remarks” of this latter species). This genus was diagnosed from Brycon View in CoL by possessing the middle caudal-fin rays elongated “into a point”. Curiously, once again, Eigenmann soon afterwards recanted and considered Triurobrycon as a synonym of Brycon ( Eigenmann, 1910: 430) View in CoL , though this generic name had some longevity in the brazilian ichthyological literature (e.g., Schubart, 1962; Godoy, 1975). A small distal elongation of the middle-caudal fin rays is in fact a common feature present in several Brycon View in CoL species. Zanata (2000) used this character as one of the synapomorphies diagnosing a well-supported monophyletic group within Brycon View in CoL , that included B. falcatus View in CoL , B. orbignyanus View in CoL , B.hilarii View in CoL , B. orthotaenia View in CoL , B. amazonicus View in CoL , B. whitei View in CoL , and B. melanopterus View in CoL .
Intrarrelationships. Howes (1982: 46) was the first to discuss possible “species assemblages” within the genus. Though these assemblages were not considered to be “monophyletic units” by that author, he proposed them “as a framework for a more rigorous analysis” ( Howes, 1982: 47). Three out of the five “species assemblages” proposed by Howes (1982) included cis-andean Brycon View in CoL species: the Brycon falcatus View in CoL -group, the Brycon acuminatus View in CoL -group, and the Brycon orbignyanus View in CoL -group.
So far, four phylogenetic analyses has been undertaken aiming to understand the intrarelationships of the genus. Hilsdorf et al. (2008) presented a hypothesis of relationships within the genus using partial 16S rDNA mitochondrial sequences of 13 Brycon species, plus the Bryconinae Henochilus wheatlandii . A more comprehensive analysis of the phylogenetic relationships of the genus, based on morphological data was undertaken by Zanata (2000), but still remains unpublished. Both hypotheses agree in identifying a well-defined, derived group that includes the large-sized, diverse-colored Brycon species widespread in the large floodplain rivers of cis-andean South America that includes B. falcatus , B. orbignyanus , B.hilarii , B. orthotaenia , B. amazonicus , B. whitei , and B. melanopterus , and which roughly corresponds to the Brycon falcatus -group of Howes (1982). Hilsdorf et al. (2008) hypothesis identifies monophyletic clades including the Brycon species of eastern Brazil ( B. opalinus , B. insignis , B. ferox , and B. nattereri ) plus Henochilus wheatlandii , and a monophyletic clade including some trans-andean species. This roughly agrees with the hypothesis of Zanata (2000), though in her hypothesis the Brycon species from eastern Brazil does not form a monophyletic clade. Recently, two additional molecular phylogenies were presented for the genus. The first, by Abe et al. (2014) presented a more comprehensive hypothesis of Brycon (including Salminus ), based on partial sequences of two mitochondrial and three nuclear genes. Abe et al. (2014) analysed 19 out of the currently 44 valid species of Brycon , plus the monotypic genera Chilobrycon and Henochilus , and all species of Salminus , and considered Brycon in the present sense as paraphyletic without the inclusion of Salminus . They recovered five monophyletic clades within Brycon , the most basal one including four of the analysed trans-andean Brycon species, plus Chilobrycon deuterodon , and then successively a clade including all Salminus species, a clade formed solely by Brycon pesu , and a clade formed by Brycon amazonicus , B. hilarii , B. gouldingi , B. orbignyanus , B. orthotaenia , and B. moorei , which is sister of a clade containing two subclades, one composed by B. falcatus and B. melanopterus , and the other including exclusively Brycon species from eastern Brazil, as well as Henochilus wheatlandii . Most of these clades seems to concur with morphological evidence, but the inclusion of Salminus within Brycon , as well as the relationships among them, are apparently at odds with the morphological evidence. The second, by Travenzoli et al. (2015), focused on the species occurring in eastern Brazil and provided additional evidence for the monophyly of a clade composed by Brycon species occurring in eastern Brazil, including Henochilus wheatlandii . The position of Henochilus wheatlandii not only within Brycon , but also within the clade of Brycon from eastern Brazil, already found by Hilsdorf et al. (2008) and confirmed by Abe et al. (2014) and Travenzoli et al. (2015), is revealing. The other monotypic genus of Bryconinae , Chilobrycon , was also recovered within the genus Brycon , both using morphological ( Zanata, 2000) and molecular ( Abe et al., 2014) data. It seems likely that both genera are synonyms of Brycon , but until both a comprehensive morphological study and a broader molecular analysis of the genus are undertaken, no changes at the generic level within Bryconinae seem advisable.
Distribution. From ríos Grijalva and Usumancita, Chiapas state, Mexico ( Miller et al., 2005) southwards to Panama in Central America ( Hildebrand, 1938), and transandean river systems, from Río Jequetepeque in northern Peru ( Pearson, 1937) and Río Tumbes at the peruvian/ecuatorian border ( Chirichigno, 1963; Géry & de Rham, 1981) (but actually occurring southward down to Río Santa, Departamento Ancash, central-northern coastal Peru; H. Ortega, pers. comm..; Brycon cf. atrocaudatus ; MUSM 2990, MUSM 3003) to the Lake of Maracaibo system, Venezuela ( Moscó Morales, 1988). At cis-andean South America, the genus Brycon is widespread across the major river systems, i.e., Río Orinoco, rio Amazonas, Rio de la Plata, and rio São Francisco basins, guyanese river systems (from Essequibo River in Guyana to Mana River in French Guiana), and small coastal river systems from the rio São Francisco mouth southwards to the rio Paraíba do Sul basin in eastern Brazil. There are no records, and the genus is with all likelihood absent, from the several rivers drainages from northeastern Brazil between the mouths of the rio São Francisco and rio Capim, and from the coastal river systems from southeastern South America situated to the south from the Baía de Guanabara in Rio de Janeiro, eastern Brazil.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Brycon Müller & Troschel
| Lima, Flávio C. T. 2017 |
Catabasis
| Eigenmann 1900: 358 |
Megalobrycon Günther, 1869 : 423
| Gunther 1869: 423 |
Megalobrycon Günther (1869: 423)
| Lima 2000: 158 |
| Howes 1982: 19 |
| Eigenmann 1910: 430 |
| Gunther 1869: 423 |
Chalcinopsis
| Kner 1863: 226 |
Brycon Müller & Troschel, 1844 : 90
| Eigenmann 1910: 430 |
| Muller 1844: 90 |